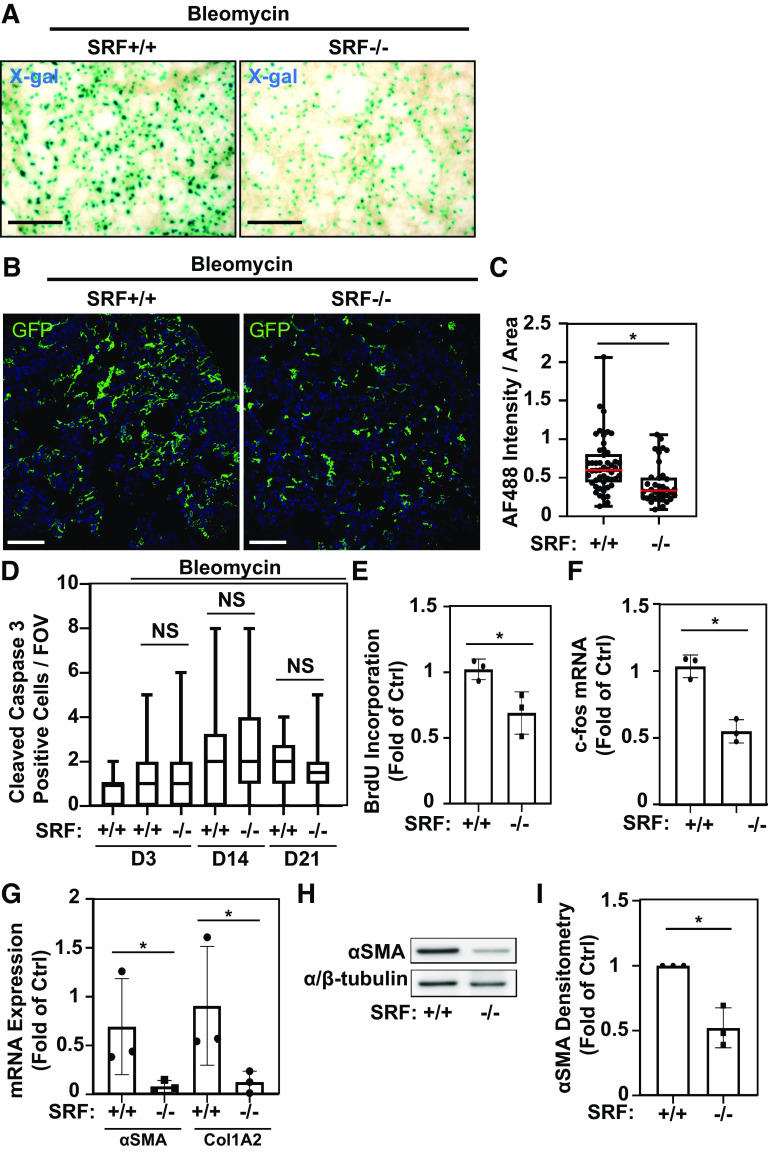
Figure 6.
Serum response factor (SRF) is essential for lung mesenchymal cell expansion, proliferation, and myofibroblast differentiation in bleomycin-induced pulmonary fibrosis. A: Tbx4LE-rtTA-LacZ/TetO-Cre/SRFf,f were administered doxycycline (2 mg/mL + 50 mg/mL sucrose in water), resulting in SRF knockdown in LMC (SRF−/−), or sham (50 mg/mL sucrose in water; SRF+/+) starting 2 days before intratracheal treatment with 1 U/kg bleomycin. Eight days after bleomycin treatment, 150-μm-thick living lung slices were stained with X-gal and imaged (representative images are displayed). B: Tbx4LE-rtTA/TetO-Cre/mTmG (SRF+/+) and Tbx4LE-rtTA/TetO-Cre/mTmG/SRFf,f (SRF−/− in LMC) mice were treated with doxycycline, and 2 days later subject to intratracheal bleomycin. Fourteen days after bleomycin instillation, lungs were fixed with 10% neutral buffered formalin, paraffin embedded, and stained against green fluorescent protein (GFP). Fluorescence images were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). C: total GFP expression per field of view (FOV, ×20 magnification) in at least 10 FOV/mouse and three mice/condition was quantified via alexa fluor 488 (AF488) quantification in the mouse lung parenchyma using ImageJ (red bar represents median value). D: Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f or Tbx4LE-rtTA/TetO-Cre/tdTom controls (SRF+/+) were administered either doxycycline (SRF−/− in LMC) or sham (SRF+/+) starting 2 days before (days 3 and 14 end points) or 5 days after (day 21 end point) intratracheal bleomycin treatment. Three, 14, or 21 days after bleomycin treatment, mouse lungs were fixed with 10% neutral buffered formalin, paraffin embedded, and subject to immunohistochemical staining against cleaved caspase 3. Cleaved caspase 3-positive cells were manually counted per FOV (×40 magnification) in at least 10 FOV/mouse and three mice/condition. E and F: Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f were administered doxycycline starting 2 days before intratracheal bleomycin treatment. Three days after bleomycin treatment, mouse lungs were minced to derive mouse lung fibroblasts (MLFs) cultures and subsequently fluorescence-activated cell sorted (FACS) against tdTom expression (to isolate tdTom-positive LMCs). tdTom-positive LMC (SRF−/−) and tdTom-negative fibroblasts (SRF+/+) were then subject to either bromodeoxyuridine (BrdU) cell proliferation assay in triplicate (E, n = 3 for each condition) or RT-PCR against c-fos and housekeeping gene (F, n = 3 including two biological replicates and one technical replicate: one cell line FAC sorted, cultured, and RT-PCR analyzed separately). Paired Student’s t test (*P < 0.05) was utilized to assess statistical significance in E and F. G: Tbx4LE-rtTA/TetO-Cre/tdTom (SRF+/+) or Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f (SRF−/− in LMC) were given doxycycline in water 2 days before intratracheal treatment with bleomycin, as indicated. Eight days after bleomycin (and 10 days after start of doxycycline) treatment, mouse lungs were digested to single-cell suspension and subjected to FACS for expression of tdTom, separating LMC from the rest of the cell populations. tdTom-positive LMCs from the two mouse groups were immediately lysed for RNA and subject to RT-PCR against smooth muscle α actin (αSMA, encoded by Acta2 gene), Collagen 1A2 (Col1A2), and housekeeping gene (n = 3 for each condition). H: Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f were treated as in E and F. Following FAC sorting, tdTom-positive LMCs and tdTom-negative fibroblast lysates were analyzed by Western blot with indicated antibodies. I: densitometry of Western blots (n = 3 for each condition) was done using ImageJ. Unless otherwise noted, unpaired Student’s t test (*P < 0.05) was utilized to assess statistical significance between groups. In all images, scale bar = 100 µm. All bar graphs represent means ± SD.