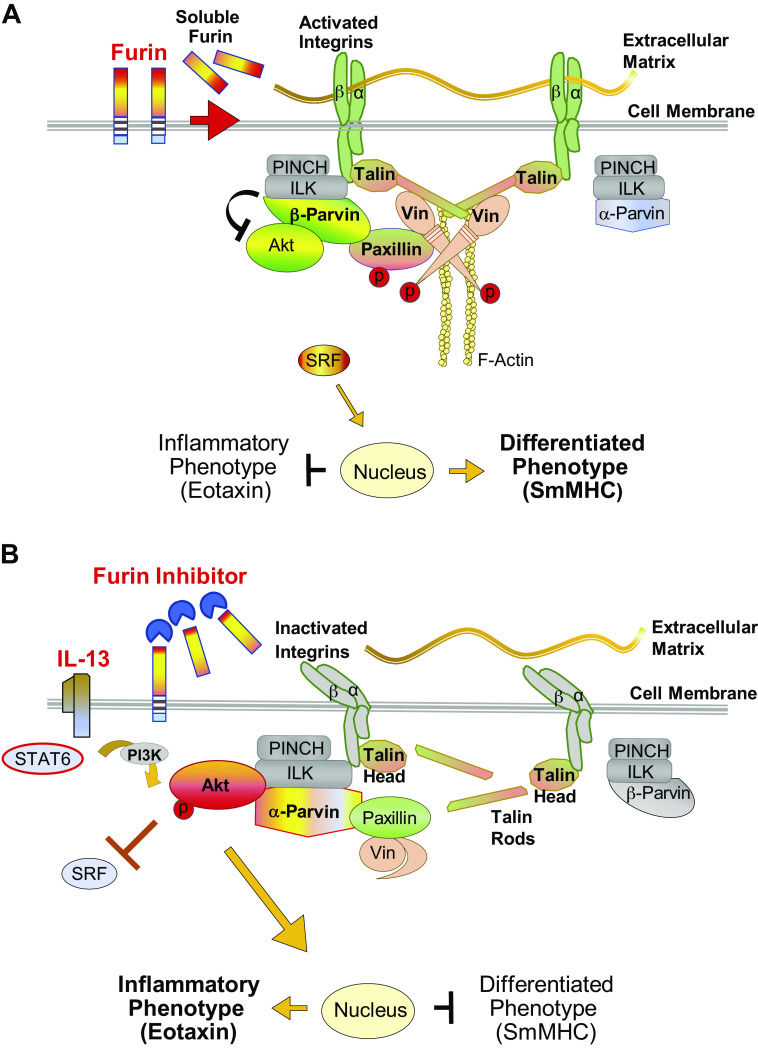
Figure 9.
Proposed mechanism for the regulation of inflammation by furin in airway smooth muscle. A: furin activates integrin-mediated signaling pathways regulating Akt activity and phenotype expression in airway smooth muscle. Furin promotes the cross linking of β integrin proteins by talin, resulting in integrin activation and the high-affinity binding of integrin proteins to the extracellular matrix. Vinculin is activated by its binding to full-length talin, facilitating phosphorylation of its binding partner paxillin. Phosphorylated paxillin binds to β-parvin IPP (ILK, PINCH, parvin) complexes, which promotes the preferential binding of Akt to β-parvin instead of integrin-linked kinase (ILK). This prevents the activation of Akt by ILK and suppresses Akt-mediated synthetic pathways that induce an inflammatory phenotype. This promotes the localization of serum response factor (SRF) to the nucleus and the expression of a differentiated phenotype. α-Parvin IPP complexes are inactive because they are not recruited by phosphorylated paxillin. B: effect of furin inhibition or IL-13 stimulation on integrin-mediated signaling pathways regulating Akt activity and phenotype expression in airway smooth muscle. Stimulation with IL-13 or treatment with furin inhibitor leads to talin cleavage, which prevents the cross linking of β integrins by talin, resulting in integrin inactivation. This prevents the high-affinity binding of integrins to the extracellular matrix. Vinculin remains in an inactive complex with paxillin because vinculin cannot be activated by cleaved talin, and paxillin phosphorylation is therefore prevented. Unphosphorylated paxillin binds to α-parvin IPP complexes, which promotes the binding of Akt directly to ILK. ILK induces Akt activation resulting in an inflammatory phenotype. SRF localization to the nucleus is inhibited by activated Akt, which suppresses the expression of a differentiated smooth muscle phenotype. β-Parvin IPP complexes remain inactive, as they are not recruited by unphosphorylated paxillin. smMHC, smooth muscle myosin heavy chain.