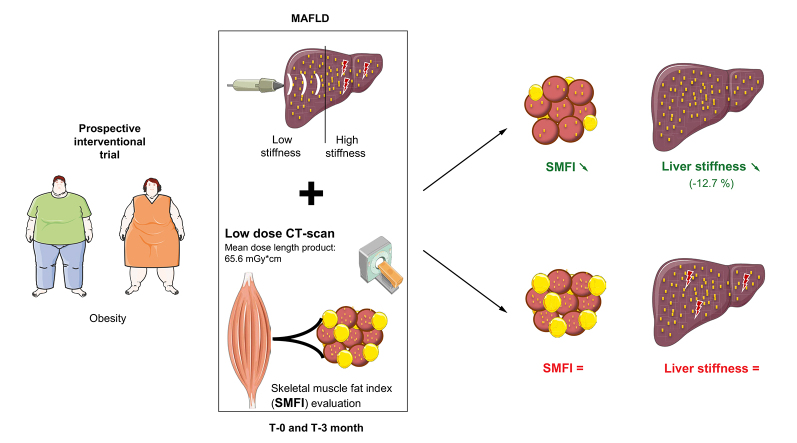
Abstract
Background & Aims
Retrospective cross-sectional studies linked sarcopenia and myosteatosis with metabolic dysfunction-associated fatty liver disease (MAFLD). Here, we wanted to clarify the dynamic relationship between sarcopenia, myosteatosis, and MAFLD.
Methods
A cohort of 48 obese patients was randomised for a dietary intervention consisting of 16 g/day of inulin (prebiotic) or maltodextrin (placebo) supplementation. Before and after the intervention, we evaluated liver steatosis and stiffness with transient elastography (TE); we assessed skeletal muscle index (SMI) and skeletal muscle fat index (SMFI) (a surrogate for absolute fat content in muscle) using computed tomography (CT) and bioelectrical impedance analysis (BIA).
Results
At baseline, sarcopenia was uncommon in patients with MAFLD (4/48, 8.3%). SMFI was higher in patients with high liver stiffness than in those with low liver stiffness (640.6 ± 114.3 cm2/ Hounsfield unit [HU] vs. 507.9 ± 103.0 cm2/HU, p = 0.001). In multivariate analysis, SMFI was robustly associated with liver stiffness even when adjusted for multiple confounders (binary logistic regression, p <0.05). After intervention, patients with inulin supplementation lost weight, but this was not associated with a decrease in liver stiffness. Remarkably, upon intervention (being inulin or maltodextrin), patients who lowered their SMFI, but not those who increased SMI, had a 12.7% decrease in liver stiffness (before = 6.36 ± 2.15 vs. after = 5.55 ± 1.97 kPa, p = 0.04).
Conclusions
Myosteatosis, but not sarcopenia, is strongly and independently associated with liver stiffness in obese patients with MAFLD. After intervention, patients in which the degree of myosteatosis decreased reduced their liver stiffness, irrespective of body weight loss or prebiotic treatment. The potential contribution of myosteatosis to liver disease progression should be investigated.
Clinical Trials registration number
Lay summary
The fat content in skeletal muscles (or myosteatosis) is strongly associated with liver stiffness in obese patients with MAFLD. After a dietary intervention, patients in which the degree of myosteatosis decreased also reduced their liver stiffness. The potential contribution of myosteatosis to liver disease progression should be investigated.
Keywords: Liver, Sarcopenia, Muscle fat, Myosteatosis, CT scan
Abbreviations: γGT, γ-glutamyl transferase; ALM, appendicular lean mass; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BIA, bioelectrical impedance analysis; BMI, body mass index; CAP, controlled attenuation parameter; CT, computed tomography; CTDIvol, volume CT dose index; DEXA, dual-energy X-ray absorptiometry; DLP, dose–length product; FFM, fat-free mass; HbA1c, haemoglobin A1c; HT, hypertension; HU, Hounsfield unit; ITF, inulin-type fructans; L3, third lumbar level; M0, baseline; M3, end of the 3-month intervention; MAFL, metabolic associated fatty liver; MAFLD, metabolic dysfunction-associated fatty liver disease; MRI, magnetic resonance imaging; NASH, non-alcoholic steatohepatitis; PMI, psoas muscle index; SMD, skeletal muscle density; SMDpsoas, psoas muscle density; SMFI, skeletal muscle fat index; SMFIpsoas, psoas fat index; SMI, skeletal muscle index; SMIbw, SMI scaled on body weight; SMIht2, SMI scaled on height squared; TE, transient elastography
Graphical abstract
Highlights
-
•
Low-radiation CT scan enables muscle evaluation (quantity and composition).
-
•
Muscle mass is not low in patients with MAFLD and high liver stiffness.
-
•
In contrast, myosteatosis is strongly associated with liver stiffness.
-
•
Lower myosteatosis after dietary intervention is associated with improved MAFLD.
Introduction
Metabolic dysfunction-associated fatty liver disease (MAFLD) is the most common chronic liver disease in the world.1 MAFLD spectrum ranges from metabolic associated fatty liver (MAFL), affecting 25% of the world adult population, to MAFLD, which may potentially lead to fibrosis, cirrhosis, and hepatocellular cancer.1 Current evidence designates advanced fibrosis as the main determinant of long-term prognosis in patients with MAFLD, as fibrosis associates with hepatic and extrahepatic complications as well as with mortality.1 Fibrosis is mainly driven by hepatocellular injury and ongoing (or chronic) inflammation.1
Sarcopenia, defined by generalised loss of skeletal muscle mass and strength (the latter data being rarely available, although recommended by international consensus2,3), has gained attention during the past decade as a predictor of disease outcome.4 Indeed, sarcopenia strongly and independently predicts complications and mortality in several chronic diseases, irrespective of the organ involved and of the aetiology.5,6 In the context of MAFLD, numerous studies report a strong association between ‘a low skeletal muscle index’ (being the muscle mass scaled on height squared, weight, or BMI) and liver fibrosis severity, even in the absence of cirrhosis.7
Muscle fat infiltration (or myosteatosis) is a pathological feature8 that frequently co-exists with sarcopenia.9,10 Myosteatosis is described in obesity, diabetes, and metabolic syndrome.8,11 Studies that investigate myosteatosis in MAFLD are scarce.[12], [13], [14] Indeed, in contrast with bioelectrical impedance analysis (BIA) or dual-energy X-ray absorptiometry (DEXA), which readily evaluates muscle mass, the assessment of myosteatosis requires computed tomography (CT) or magnetic resonance imaging (MRI),15 infrequently performed in cohorts of individuals with MAFLD.
Inulin-type fructans (ITF) have been shown to be beneficial in the context of obesity,16 a condition highly associated with MAFLD.17 Between 2016 and 2018, we conducted a multicentre intervention trial in obese patients presenting comorbidities in the context of the Food4Gut.18 This clinical intervention (a randomised, single-blinded, and placebo-controlled trial) aimed to evaluate the impact on metabolic comorbidities of 16 g/day native inulin (prebiotic group) vs. maltodextrin (placebo group), coupled to dietary advice to consume inulin-rich (for the prebiotic group) vs. inulin-poor (for placebo) vegetables during 3 months, in addition to 30% caloric restriction.18 In this trial, inulin supplementation was associated with a greater weight loss and substantial improvement of metabolic parameters, but the effects on skeletal muscle have not been investigated.18
In a sub-cohort of the Food4Gut trial, we aimed at clarifying the dynamic relationship between sarcopenia, myosteatosis, and MAFLD severity as reflected by liver stiffness. We first developed a low-dose CT scan protocol dedicated to the analysis of skeletal muscle mass and fat content evaluation. We then evaluated muscle parameters as well as liver stiffness before and after the dietary intervention consisting of a caloric restriction diet with prebiotic (inulin) or placebo (maltodextrin) supplementation.
Methods
Patients cohort and dietary intervention
Patients who participated to a multicentric randomised placebo-controlled trial and who were recruited in the single centre of Saint-Luc hospital were selected for this study (Fig. S1).18 The inclusion criteria were as follows: BMI >30 kg/m2, aged from 18 to 65 years, Caucasian ethnicity, presence of at least 1 obesity-related metabolic disorder (prediabetes/diabetes, dyslipidaemia, hypertension (HT), elevated alanine aminotransferase [ALT]/aspartate aminotransferase [AST]/γ-glutamyl transferase [γGT] suggestive of MAFLD). The exclusion criteria included the use of antibiotics and probiotics/prebiotics and ongoing diets (e.g. high-protein, high-fibre diet) within 6 weeks of enrolment, excessive alcohol consumption (more than 3 glasses/day), type 1 diabetes, or other cause of chronic liver disease (e.g. viral hepatitis and genetic disease). Following the screening, all participants were randomised to consume either 16 g/day of native inulin (extracted from chicory root, Cosucra, Belgium) or 16 g/day of maltodextrin (Cargill, Belgium), provided in an identical packaging, for a total duration of 3 months. The participants met a dietician before and monthly during the intervention. At baseline, the dietician calculated energy expenditure of the participants in order to prescribe a hypocaloric diet corresponding to −30% of the calculated energy expenditure. In addition, all participants received a cookbook with recipes based on vegetables either rich or poor in fructans according to the inulin vs. placebo randomisation and were advised to consume at least 1 meal proposed in the cookbook per day.
This study was approved by the ‘Comité d’éthique Hospitalo-facultaire de Saint-Luc’. Written informed consent was obtained in compliance with the European law 2001/20/CE guidelines from all participants before inclusion. The authors ensure that the study has been carried out in accordance with the ethical guidelines set out in the Declaration of Helsinki. The trial protocol was published on protocols.io (dx.doi.org/10.17504/protocols.io.baidica6), and the trial was registered at ClinicalTrials.gov under identification number NCT03852069. At baseline, a total of 48 patients were considered based on the concomitant availability of liver and muscle evaluation with transient elastography (TE) and CT scan, respectively (Fig. S1). All patients (n = 48) underwent dietary intervention for a period of 3 months, but 35 had TE and CT scan available after this nutritional intervention (inulin n = 16 and maltodextrin n = 19) and thus were included to evaluate the effects of the intervention (Fig. S1).
Evaluation of metabolic and liver parameters
Metabolic and blood parameters such as weight, height, waist circumference, glycaemia, haemoglobin A1c (HbA1c), AST, ALT, γGT, and lipid profile were measured at baseline (M0) and after 3 months of intervention (M3). Liver stiffness and controlled attenuation parameter (CAP) measurements were performed using TE (FibroScan®, Echosens, Paris, France) by 1 single experienced operator (NL).19 Patients were stratified based on the result of the CAP and liver stiffness measurements, as previously described[19], [20], [21] and detailed in our initial analysis.22 A liver stiffness ≥7.8 kPa if measured with the M probe or ≥6.4 kPa if measured with the XL probe was defined as ‘high liver stiffness’.21 In the context of MAFLD, the cut-off of 296 dB/m was used to define severe steatosis.20
Evaluation of skeletal muscles
On the day of TE analysis, muscle mass was assessed by bio-impedance devices (BIA 101, Akern, Italy; Biocorpus, Medi Cal, Germany; Tanita BC-418 MA, Tanita, UK), and an abdominal CT scan at the third lumbar level (L3) was performed to measure muscle mass and density indexes. Of note, we used a dedicated low-dose protocol centred at the L3 and evaluated the induced volume CT dose index (CTDIvol) and the dose–length product (DLP). We used Hounsfield unit (HU) values at the commonly accepted threshold of −29 to +150 HU23 to semi-automatically delineate psoas, dorsal, and abdominal muscles. Muscle area and density were quantified by the Slice-O-Matic software, version 4.3 (TomoVision, Montreal, Canada). Total muscle area was normalised for stature and was referred to as the skeletal muscle index (SMI) (cm2/m2). Sarcopenia was defined as a SMI <41 cm2/m2 in female and <53 cm2/m2 in male.24 We measured the mean skeletal muscle density (SMD), and the absolute amount of fat in the muscle was computed as the ratio of the muscle area in cm2 by muscle density in HU. This ratio, multiplied by 100, is referred to as the skeletal muscle fat index (SMFI). For simplification, the term “myosteatosis” will be used to denote a high(er) SMFI or absolute muscle fat content. All CT images were analysed by a single operator (MN), unaware of metabolic and TE data.
Statistics
Data are presented as mean ± SD unless specified otherwise. Statistical analyses were performed using 2-tailed Student’s t test (equal variance) or Welch’s t test (unequal variance) or 2-way ANOVA followed by Bonferroni’s post hoc test using GraphPad Prism 8 software. Multivariate analysis were performed on SPSS (v24) using binary logistic regression, linear regression, and a repeated-measures t test after intervention. Correlation was computed using Pearson’s or Spearman’s coefficients (if ordinal variables or normality assumption rejected per Shapiro–Wilk’s test). All parameters were systematically checked for collinearity. Differences were considered significant at values of p <0.05.
Results
A low-dose CT protocol enables muscle evaluation with a limited amount of radiation
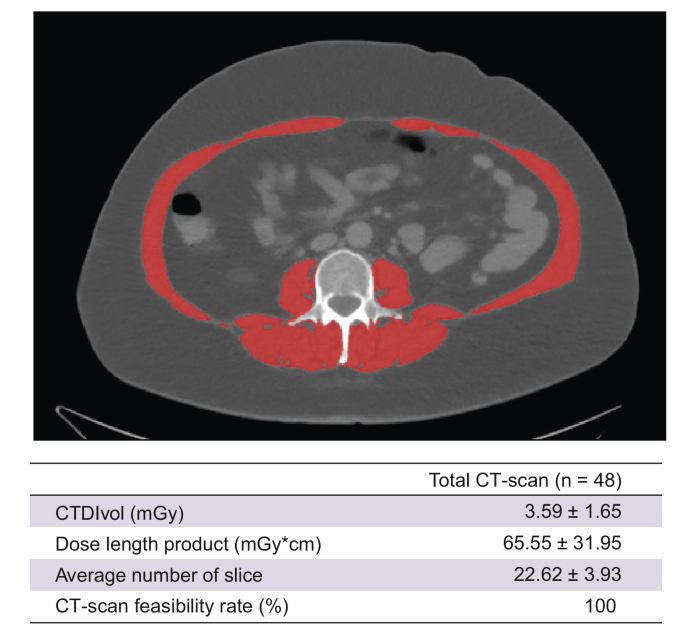
CT images at L3 is internationally recognised as the imaging gold standard to evaluate skeletal muscle mass and density.2 We first evaluated the radiation exposure of a low-dose protocol dedicated to muscle evaluation (Fig. 1). CTDIvol was 3.59 ± 1.65 mGy, and mean DLP was 65.55 ± 31.95 mGy∗cm. CT scan was feasible in 100% of patients (48/48) (Fig. 1), and CT images were successfully used to measure muscle area (i.e. combination of psoas, erector spinae, quadratum lumborum, oblique, and rectus) and densities (HU) (see below).
Fig. 1.
Feasibility of a low-dose CT scan acquisition to measure body composition.
(Top) Illustration of L3 centred abdominal slice to measure skeletal muscle area. (Bottom) Radiation data induced by low-dose CT protocol. CT, computed tomography; CTDIvol, volume CT dose index; L3, third lumbar level.
Sarcopenia is uncommon in obese patients with MAFLD
Baseline characteristics of the 48 obese patients are shown in Table 1. Mean BMI was 36 ± 6 kg/m2. Severe steatosis was found in 35 patients, and 12 had high liver stiffness (2 female and 10 male). Mean BIA–fat-free mass (BIA-FFM) was 67.0 ± 13.1 kg, BIA–SMI scaled on body weight (BIA-SMIbw) was 63.2% ± 8.4%, and BIA-SMI scaled on height squared (BIA-SMIht2) was 22.6 ± 3.2 kg/m2. Mean CT-SMI was 59.5 ± 11.9 cm2/m2. Four out of 48 patients (8.3 %) had CT-defined sarcopenia (i.e. CT-SMI <41 cm2/m2 and <53 cm2/m2 in female and male, respectively).24 Among them, only 1 had high liver stiffness. Hence, sarcopenia was uncommon in this cohort of obese patients and was not associated with high liver stiffness.
Table 1.
Characteristics of obese patients with MAFLD before intervention.
| Total patients (n = 48) | |
|---|---|
| Age (years) | 50 ± 11 |
| BMI (kg/m2) | 36 ± 6 |
| Female sex (%) | 24 (50%) |
| Alanine aminotransferase (U/L) | 40 ± 25 |
| Aspartate aminotransferase (U/L) | 26 ± 12 |
| CAP (dB/m) | |
| Mild steatosis | 265 ± 26 (n = 13) |
| Severe steatosis | 347 ± 27 (n = 35) |
| Liver stiffness (kPa) | |
| Low stiffness | 5.0 ± 1.3 (n = 36) |
| High stiffness | 10.4 ± 3.0 (n = 12) |
| BIA–fat-free mass (kg) | 67.0 ± 13.1 |
| BIA-SMIbw (%) | 63.2 ± 8.4 |
| BIA-SMIht2 (kg/m2) | 22.6 ± 3.2 |
| Skeletal muscle index (cm2/m2) | 59.5 ± 11.9 |
| Skeletal muscle density (HU) | 32.9 ± 6.5 |
| Patients with sarcopenia (%) | 4 (8.3%) |
BIA, bioelectrical impedance analysis; BIA-SMIbw, BIA–SMI scaled on body weight; BIA-SMIht2, BIA–SMI scaled on height squared; CAP, controlled attenuation parameter; HU, Hounsfield unit; MAFLD, metabolic dysfunction-associated fatty liver disease; SMI, skeletal muscle index.
Increasing MAFLD severity is associated with a higher rather than a lower muscle mass
Disconcerted by the low prevalence of sarcopenia in this cohort, we next questioned whether patients with MAFLD and high liver stiffness had a lower muscle mass than had those with low liver stiffness. We computed CT-SMI, CT-psoas muscle index (CT-PMI), CT-based whole-body FFM and appendicular muscle mass derived from validated formulae,25 BIA-derived appendicular muscle mass index scaled on body weight (SMIbw) or height squared (BIA-SMIht2) and stratified the patients according to liver steatosis and stiffness (Table 2).
Table 2.
MAFLD severity is associated with a higher rather than a lower muscle mass.
| Muscle mass indexes | Mild steatosis (n = 35) | Severe steatosis (n = 13) | Δ | p value | Low liver stiffness (n = 36) | High liver stiffness (n = 12) | Δ | p value |
|---|---|---|---|---|---|---|---|---|
| CT–skeletal muscle index (cm2/m2) | 53.3 ± 13.1 | 61.9 ± 10.7 | +16.0% | 0.025 | 58.0 ± 11.6 | 64.2 ± 11.4 | +12.7% | 0.115 |
| CT–psoas muscle mass index (cm2/m2) | 6.8 ± 2.4 | 8.2 ± 2.0 | +19.6% | 0.059 | 7.6 ± 2.3 | 8.3 ± 1.8 | +9.4% | 0.367 |
| CT–whole body fat-free mass (kg) | 52.7 ± 13.6 | 60.8 ± 11.4 | +15.4% | 0.043 | 56.1 ± 12.2 | 66.0 ± 10.1 | +17.8% | 0.016 |
| CT–appendicular skeletal muscle/height2 (kg/m2) | 7.0 ± 1.4 | 8.0 ± 1.2 | +14.2% | 0.025 | 7.5 ± 1.3 | 8.2 ± 0.3 | +9.3% | 0.151 |
| BIA–fat-free mass (kg) | 62.4 ± 12.7 | 68.4 ± 12.9 | +9.7% | 0.190 | 63.5 ± 12.2 | 76.7 ± 10.8 | +19.5% | 0.002 |
| BIA-SMIbw (%) | 62.3 ± 9.5 | 63.6 ± 8.1 | +2.1% | 0.691 | 62.8 ± 8.7 | 64.4 ± 7.6 | +2.3% | 0.556 |
| BIA-SMIht2 (kg/m2) | 20.1 ± 2.6 | 23.1 ± 3.2 | +10.4% | 0.045 | 21.9 ± 2.5 | 24.7 ± 4.0 | +13.8% | 0.006 |
CT–skeletal muscle index: whole muscle area at L3 divided by height2; CT–psoas muscle mass index: psoas muscle area at L3 divided by height2; CT–fat-free mass: whole body fat free mass derived from the prediction model (see Methods); CT–height-scaled appendicular muscle mass index: CT–estimated appendicular muscle mass divided by height2; BIA–fat-free mass: fat-free mass derived from BIA; BIA-SMIbw: BIA–body weight-scaled skeletal muscle index: BIA–fat-free mass × 100 divided by body weight; BIA-SMIht2: BIA–height-scaled skeletal muscle index: BIA–fat-free mass divided by height2. Δ: percentage change between means. Student’s t test was used to compare means. Values in bold indicate statistical significance. BIA, bioelectrical impedance analysis; CT, computed tomography; L3, third lumbar level.
CT-SMI and CT-FFM were higher in patients with severe steatosis (+16%, p = 0.025, and +15.4%, p = 0.043, respectively) or with high liver stiffness (+12.7%, p = 0.115 and +17.8%, p = 0.016, respectively). BIA-SMIht2 was higher in patients with severe steatosis (+10.4%, p = 0.045) or high liver stiffness (+13.8%, p = 0.006) than in those with mild steatosis and low liver stiffness, respectively. Of note, we found a high correlation between CT-SMI and BIA-SMIht2 (Table S1). Thus, obese patients with severe MAFLD (i.e. severe steatosis or high liver stiffness) have a larger – rather than an expected lower – muscle mass.
Rationale behind SMFI use and application to obese patients with high liver stiffness
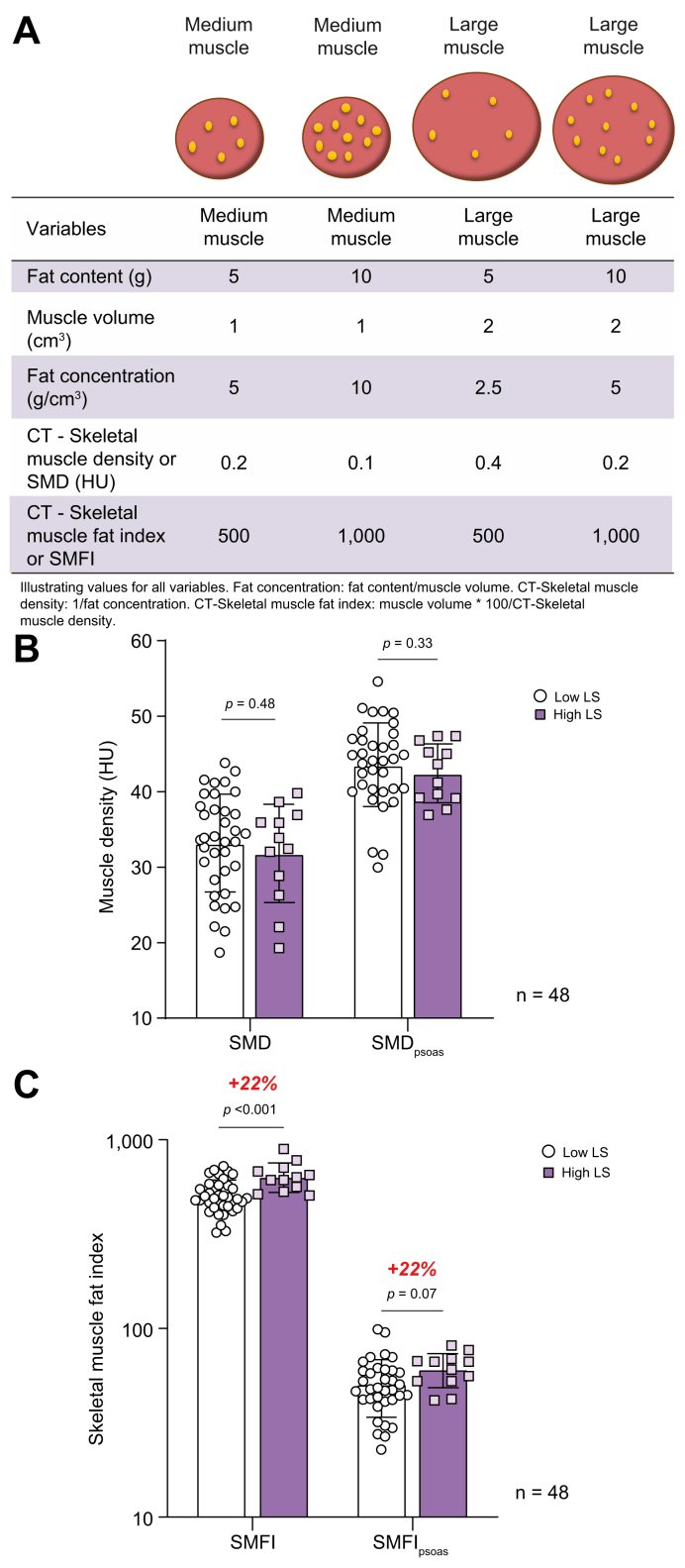
We then evaluated whether other muscle features were associated with high liver stiffness. CT-based SMD (in HU) is inversely related to muscle lipid concentration26 and is commonly used as a marker of myosteatosis. However, in the context of significant muscle hypertrophy (as shown above), the absolute lipid content, rather than the lipid concentration, might better describe lipid infiltration. Thus, we considered SMFI, wherein muscle area is divided by muscle density, as we have recently described in a large cohort of obese patients.27 As shown in Fig. 2A, SMFI better reflects on the total amount of fat in muscle than muscle density and is particularly relevant to appreciate fat content if muscle mass differs according to disease status.
Fig. 2.
Rationale for SMFI development.
(A) Illustration of rationale behind SMFI development; (B) whole SMD and SMDpsoas; and (C) SMFI and SMFIpsoas in patients with low LS vs. those with high LS (2-tailed Student’s t test, n = 48). All data are mean ± SD. Significant differences are considered at p <0.05. CT, computed tomography; HU, Hounsfield unit; LS, liver stiffness; SMD, skeletal muscle density; SMDpsoas, psoas muscle density; SMFI, skeletal muscle fat index; SMFIpsoas, psoas fat index.
We separated the cohort according to liver stiffness measurements. There was a trend for lower whole SMD and lower psoas muscle density (SMDpsoas) in patients with high liver stiffness than in those with low liver stiffness (Fig. 2B). The trend remained upon stratification for sex (Fig. S2B). Remarkably, SMFI whether in whole skeletal muscle at L3 (SMFI) as well as in psoas alone (SMFIpsoas) was 22% higher in patients with high liver stiffness than in those with low liver stiffness (p <0.001 and p = 0.07 for SMFI and SMFIpsoas, respectively) (Fig. 2C). The difference in SMFI between patients with high and low liver stiffness was larger in females (+34%) than in males (+11%) (Fig. S2B). When SMFI data were grouped according to sex-specific quartile (Fig. S2C), none (0/12) of the patients in the lowest SMFI quartile (Q1) had high liver stiffness, whereas 50% (6/12) of patients in the highest SMFI quartile (Q4) had high liver stiffness. Hence, the prevalence of high liver stiffness is greater in patients with myosteatosis.
SMFI is a significant predictor of liver stiffness
We found a significant correlation between liver stiffness and SMFI (Fig. S3) and thus sought to determine whether SMFI was a significant predictor of liver stiffness. We applied a linear regression model adjusted for age, sex, visceral obesity (waist circumference), and probe type. SMFI was the only significant predictor of liver stiffness (p = 0.032) (Table S2).
The association between myosteatosis and liver stiffness is independent of age, sex, liver steatosis, ALT, HbA1c, and hypertension
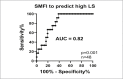
To determine whether the relationship between myosteatosis (evaluated with SMFI) and liver stiffness is robust, we used multivariate analysis to account for potential metabolic confounders (Table 3). The relationship remained significant when the models were adjusted for sex, age, liver steatosis, ALT, and HbA1c level and HT status. When considered alone, SMFI had a high power to predict high liver stiffness (AUROC = 0.82, 95% CI 0.70–0.94) (Table 3). These data support a strong and independent relationship between SMFI and liver stiffness.
Table 3.
The association between SMFI and high LS is independent from age, sex, liver steatosis, ALT, HbA1c, or HT.
| Binary logistic regression | Parameters | p value for SMFI |
|---|---|---|
| Unadjusted | — | 0.004 |
| Age, sex adjusted | Age, sex | 0.017 |
| Multivariate model 1 | Age, sex, liver steatosis (CAP) | 0.035 |
| Multivariate model 2 | Age, sex, liver steatosis (CAP), ALT | 0.032 |
| Multivariate model 3 | Age, sex, liver steatosis (CAP), ALT, HbA1c | 0.029 |
| Multivariate model 4 | Age, sex, liver steatosis (CAP), ALT, HbA1c, HT | 0.048 |
| Multivariate model 5 | Age, sex, liver steatosis (CAP), ALT, HbA1c, HT, BMI | 0.072 |
 | ||
Multivariate analysis performed using binary logistic regression. ALT, alanine aminotransferase; CAP, controlled attenuation parameter; HbA1c, haemoglobin A1c; HT, hypertension; LS, liver stiffness; SMFI, skeletal muscle fat index.
Inulin supplementation causes a decrease in body weight and muscle density but does not affect liver stiffness
Patients (n = 48) received dietary instructions to be followed for 3 months and were randomly assigned to maltodextrin or inulin supplementation. In 35 out of the 48 patients, a CT scan was available at M3 (16 in the inulin group and 19 in the maltodextrin group). We analysed muscle and liver changes between M0 and M3 (Table S3). Patients with inulin supplementation lost weight significantly (Fig. S4A), tended to lower their muscle mass (Fig. S4B), and decreased their muscle density significantly (Fig. S4C) when compared with those supplemented in maltodextrin in whom the changes were not significant. The intervention (diet and supplementation with maltodextrin or with inulin) had no effect on SMFI (Fig. S4D), liver steatosis (Fig. S4E), or liver stiffness (Fig. S4F). Of note, liver stiffness was positively correlated with ALT and AST, whether at M0 or M3 (Fig. S5).
Patients in whom the intervention reduced the SMFI also had a decrease in liver stiffness
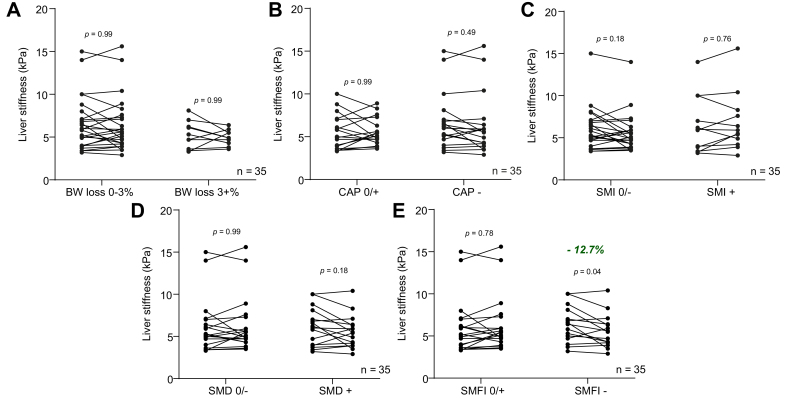
We then analysed the data the other way around and stratified patients according to changes in metabolic or muscle parameters and evaluated the associated changes in liver stiffness (Fig. 3), regardless of the dietary supplementation. Liver stiffness was unchanged in patients who experienced weight loss, reduction in liver steatosis, increase in muscle mass, or increase in muscle density from M0 to M3 (Fig. 3A–D). Remarkably, liver stiffness significantly decreased (−12.7%, p = 0.04) in patients who experienced a decrease in SMFI (Fig. 3E). This finding was not explained by intervention allocation (Fig. S6A) or differences in anthropometrical, body composition or liver parameters at M0 (Table S4). There was a significant correlation between the change (i.e. delta M0–M3) in SMFI and the change in liver stiffness (r = 0.40, p = 0.012) (Fig. S6B). We therefore evaluated the factors associated with decreased SMFI (Table 4). The patients with decreased SMFI decreased their body weight and liver steatosis, had a stable muscle mass, and significantly increased their muscle density. Hence, these patients had a decreased muscle lipid concentration (i.e. reflected by the increase of muscle density), whereas their muscle mass remained stable, which is compatible with a decrease of absolute lipid content and reflected by a decreased SMFI. Taken together, these results support a highly dynamic association between myosteatosis (evaluated here by SMFI that captures the absolute lipid content in muscle) and liver stiffness.
Fig. 3.
Patients with improved SMFI had decreased liver stiffness.
Liver stiffness changes when patients were stratified according to (A) BW loss degree (0–3% vs. >3%), (B) liver steatosis changes measured with CAP (unchanged or increased = CAP 0/+, decreased = CAP −), (C) SMI changes (decreased = SMI −, unchanged or increased = SMI 0/+), (D) SMD changes (unchanged or decreased = SMD 0/−, increased = SMD +), and (E) SMFI changes (unchanged or decreased = SMFI 0/−, increased = SMFI +) (paired sample t test, n = 35). All data are mean ± SD. Significant differences are considered at p <0.05. BW, body weight; CAP, controlled attenuation parameter; SMD, skeletal muscle density; SMFI, skeletal muscle fat index; SMI, skeletal muscle index.
Table 4.
Factors associated with decreased SMFI.
| SMFI-(n = 15) |
||||
|---|---|---|---|---|
| M0 | M3 | Δ | p value | |
| Body weight (kg) | 108.05 ± 19.04 | 106.25 ± 19.18 | −1.7% | 0.016 |
| Skeletal muscle index (cm2/m2) | 59.41 ± 13.86 | 57.37 ± 12.70 | −3.5% | 0.161 |
| Skeletal muscle density (HU) | 30.74 ± 7.00 | 32.55 ± 6.54 | +5.6% | 0.026 |
| CAP (dB/m) | 341.00 ± 41.08 | 328.40 ± 45.87 | −3.8% | 0.059 |
A repeated-measures t test was used to compare mean differences. Values in bold indicate statistical significance. CAP, controlled attenuation parameter; HU, Hounsfield unit; M0, baseline; M3, end of the 3-month intervention; SMFI, skeletal muscle fat index.
Discussion
The relationship between skeletal muscle and liver status in chronic liver diseases (and in particular in MAFLD) is a hot topic. In this pilot prospective interventional study, we used a low-dose CT scan protocol centred on L3 to evaluate skeletal muscle.25 L3-centered images for muscle assessment are usually derived from abdominal CT scan performed in standard care, for example, in patients with cancer5 or cirrhosis.9 Abdominal CT scan might be deemed unacceptable if only for the purpose of muscle evaluation, notably because of the high effective radiation dose induced to the patient (approximatively 10 mSv,28,29 a dose ≃3 years’ cumulation of natural background radiation). The dedicated low-dose protocol we used here had a mean DLP of 65.55 mGy∗cm and induced an effective radiation dose that approximates to 1 mSv,30 a dose 10 times lower than that for a standard abdominal CT scan28,29 and equivalent to that for a standard abdominal plain film.28 This protocol is thus safely applicable in clinical trials for metabolic diseases.
We found, in contrast with the current literature,31 that sarcopenia was uncommon in obese patients with MAFLD. In addition, patients with severe MAFLD (severe steatosis or high liver stiffness) also had a higher or similar muscle mass – but certainly not a lower muscle mass – when compared with those with non-severe MAFLD. In studies exploring the muscle compartment in cohorts of individuals with MAFLD, muscle mass is commonly scaled on weight or BMI instead of height.14,32 However, it is now well known that the association between sarcopenia and MAFLD is highly dependent on the scaling methodology.33 Bearing in mind that obesity per se is associated with MAFLD,1 the alleged ‘relative muscle mass and/or strength’ described in patients with MAFLD might therefore merely reflect the association between MAFLD and obesity. The gold standard CT-based SMI used here levels out this concern as SMI is scaled on height as per international consensus2,3 and thus is not distorted or tampered by obesity. Hence, our data support that, irrespective of the BIA, DEXA, CT, or MRI methodology used, raw and/or height-scaled SMI should be systematically reported, especially so in cohorts in which the prevalence of overweight or obesity is high. This could help avoid hasty and incorrect conclusions and put to rest the erroneous but now dogmatic association between sarcopenia and MAFLD. To further re-enforce this view, Chen et al.34 recently reported in 2,249 patients a lower weight-scaled SMI (26.1% vs. 27.4%, p <0.0001) but a higher total FFM and appendicular lean mass (ALM) (52.0 vs. 46.7 kg and 23.2 vs. 20.8 kg, respectively, p <0.0001), thus a higher muscle mass in patients with MAFLD when compared with those without MAFLD. A plausible explanation for the increased muscle mass seen in obese patients with MAFLD is that the mechanical overload imposed by excessive body weight increases anabolic (and eventually decreases catabolic) stimuli within the skeletal muscle.35 This scenario might hold true until liver disease becomes critical and systemic catabolic and/or anti-anabolic stimuli become predominant.
The most sticking finding in our study is that myosteatosis, that is, total fat content in muscle, is dynamically associated with liver stiffness. A potential association between fat infiltration within the skeletal muscle and MAFLD was reported by other groups[12], [13], [14] and in a large retrospective study in obese patients.27 The initial analysis of our cohort of patients supports these findings,22 yet the relationship between myosteatosis and MAFLD and its evolution over time have been poorly investigated so far. Myosteatosis translates 2 plausible biological substratum: (1) the lipid concentration, evaluated with CT-based density and expressed in HU, and (2) the absolute lipid content in the muscle compartment (as defined by the SMFI). It is univocally accepted that a high muscle lipid concentration (hence low muscle density) is associated with complications and poor prognosis in many end-stage diseases.10,36,37 However, whether absolute increase in lipid content in the muscle compartment is clinically relevant is unknown. Here, in the context of obesity-associated muscle hypertrophy, a significant increase of muscle lipid content may not necessarily translate into a lower muscle density.27 Indeed, we observed a higher SMFI (and thus a higher lipid content) in muscles of patients with high liver stiffness when compared with those with low liver stiffness. This relationship was robust and persisted in multivariate analysis when multiple confounders were accounted for. Hence, myosteatosis as evaluated per SMFI is strongly associated with liver stiffness.
The data derived from the intervention experiment remarkably strengthen our key points. We first observed that a modest diet-induced weight loss might be associated with a significant decrease of muscle mass.38 This illustrates the potential hurdle of weight loss in obese patients that have a low muscle mass at baseline. Although rare in our cohort (8.3%), patients with sarcopenic obesity can represent up to 35% of patients with end-stage liver disease.39 In addition, we highlight that in patients who maintained or gained muscle mass although they lost weight, there was no decrease of liver stiffness. Therefore, changes in muscle mass does not anticipate liver stiffness improvement. By contrast, patients that decreased SMFI had a decreased liver stiffness, irrespective of dietary supplementation. In other terms, changes in absolute lipid content (i.e. myosteatosis) within skeletal muscle are inversely associated with changes in liver stiffness. Why loss of weight was associated with decreased myosteatosis in some patients and not in others is, however, unclear. In our patients, decreased SMFI is associated with decreased/maintained muscle mass but increased muscle density, hence decreased absolute fat content. These changes were seen in a relatively short period of time (3 months), suggesting that the relationship between myosteatosis and liver stiffness is highly dynamic.
It is very tempting to speculate that myosteatosis could play a role in liver disease pathophysiology. To this end, we recently found that myosteatosis, but not sarcopenia, was a strongly associated with non-alcoholic steatohepatitis (NASH) in preclinical models.40 Thus, these models will be of great help to decipher whether a causal link between myosteatosis and MAFLD severity exists. Also, whether our observations hold true in normal/overweight patients needs to be determined.
This study has several strengths. First, to the best of our knowledge, it is the first prospective interventional study that evaluated the muscle compartment with gold standard CT scan in patients with MAFLD. Second, we developed a low-dose CT scan protocol dedicated to muscle evaluation with a very limited amount of radiation. Third, liver disease was evaluated with TE, a widely validated methodology.41 Finally, a limited number of patients and intervention duration were sufficient to unravel a significant relationship between SMFI and liver stiffness at baseline and after intervention. Some limitations have to be acknowledged. First, we had a relatively low number of patients with high liver stiffness that were not sex balanced. Nonetheless, multivariate analyses, sex-specific quartile stratification, and the intervention experiment strongly support our hypothesis (i.e. that myosteatosis is strongly associated with liver stiffness). Second, there were no patients without MAFLD. Hence, we are unable to define what is a non-pathological SMFI. However, our key point is that absolute muscle fat quantity evaluated by SMFI is reflective of liver disease progression and regression within the MAFLD spectrum. Replication studies in large cohorts will be needed to define pathological cut-off. Third, the improvement in liver stiffness in patients that decreased SMFI after intervention was mild, but we believe that in those patients with non-severe fibrosis, the decreased in liver stiffness might reflect on decreased ‘MAFLD severity’ (i.e. inflammation, ballooning, and fibrosis scores) rather than on reduction of fibrosis alone.42,43 Further studies with liver biopsies will be needed to determine the relative proportion of liver stiffness changes attributable to each parameter and their respective relationship with SMFI modulation. Fourth, muscle strength was not evaluated. Taken together, our data build on the growing rationale for large prospective preclinical and clinical studies to investigate the muscle–liver axis in MAFLD pathogenesis and treatment.
In conclusion, myosteatosis, but not sarcopenia, is strongly and independently associated with liver stiffness in MAFLD obese patients. After the nutritional intervention, the patients presenting a decreased degree of myosteatosis exhibited a reduced liver stiffness irrespective of body weight loss or prebiotic treatment. These data lay the foundation for investigating the potential role of myosteatosis as a contributor to liver disease progression and perhaps as a novel therapeutic target in MAFLD.
Financial support
This study was supported by the Service Public de Wallonie (FOOD4GUT project, convention 1318148). NMD is a recipient of grants from the Fonds de la Recherche Scientifique (FRS-FNRS, Belgium, PINT-MULTI R.8013.19 [NEURON-ERANET, call 2019] and PDR T.0068.19) and from the Fédération Wallonie-Bruxelles (Action de Recherche Concertée ARC18-23/092). PDC is a senior research associate at FRS-FNRS, supported by the Fonds Baillet Latour (Grant for Medical Research 2015) and WELBIO-CR-2019C-02R. MN has a Ph.D. fellowship grant from FRIA (FNRS, Belgium) (n°31618719). NL has a mandate as a Clinical Researcher from the FNRS (Fonds de la Recherche Scientifique, Belgium).
Authors’ contributions
Conceived and designed the dataset analysis: MN, NL, JR, J-PT, ND. Recruited the patients and carried out data collection in human studies: SH, J-PT, NL, AMN, BDP, JR. Analysed and interpreted the data: MN, NL, J-PT, JR, ND. Supervised computed tomography protocol: PT. Took charge of the administrative part of the study: AMN, JR, NMD, J-PT. Critically discussed study design, data, and manuscript for scientific content: All the authors. Wrote the original manuscript: MN, NL, ND. Contributed to the manuscript and reviewed and edited it: MN, NL, JR, AMN, PDC, LBB, BDP, PT, J-PT, NMD. Supervised the overall project and funding: ND.
Data availability statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Conflicts of interest
The authors declare that they have no conflict of interest related to this study.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank Celine Bugli and Lieven Desmet (Louvain Institute of Data Analysis and Modelling in Economics and Statistics, Louvain-la-Neuve, Belgium) for their assistance for statistical analyses. We thank Vincent Poty for his assistance in computed tomography experiments.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100323.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Sanyal A.J. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2019;16:377–386. doi: 10.1038/s41575-019-0144-8. [DOI] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Studenski S.A., Peters K.W., Alley D.E., Cawthon P.M., McLean R.R., Harris T.B. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft A.J., Sayer A.A. Sarcopenia. Lancet. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 5.Prado C.M., Purcell S.A., Alish C., Pereira S.L., Deutz N.E., Heyland D.K. Implications of low muscle mass across the continuum of care: a narrative review. Ann Med. 2018;0:1–39. doi: 10.1080/07853890.2018.1511918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazemi-Bajestani S.M.R., Mazurak V.C., Baracos V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol. 2016;54:2–10. doi: 10.1016/j.semcdb.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Bhanji R.A., Narayanan P., Allen A.M., Malhi H., Watt K.D. Sarcopenia in hiding: the risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology. 2017;66:2055–2065. doi: 10.1002/hep.29420. [DOI] [PubMed] [Google Scholar]
- 8.Hausman G.J., Basu U., Du M., Fernyhough-Culver M., Dodson M.V. Intermuscular and intramuscular adipose tissues: bad vs. good adipose tissues. Adipocyte. 2014;3:242–255. doi: 10.4161/adip.28546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montano-Loza A.J., Angulo P., Meza-Junco J., Prado C.M.M., Sawyer M.B., Beaumont C. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7:126–135. doi: 10.1002/jcsm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nardelli S., Lattanzi B., Merli M., Farcomeni A., Gioia S., Ridola L. Muscle alterations are associated with minimal and overt hepatic encephalopathy in patients with liver cirrhosis. Hepatology. 2019;70:1704–1713. doi: 10.1002/hep.30692. [DOI] [PubMed] [Google Scholar]
- 11.Miljkovic I., Kuipers A.L., Cvejkus R., Bunker C.H., Patrick A.L., Gordon C.L. Myosteatosis increases with aging and is associated with incident diabetes in African ancestry men. Obes (Silver Spring) 2016;24:476–482. doi: 10.1002/oby.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitajima Y., Eguchi Y., Ishibashi E., Nakashita S., Aoki S., Toda S. Age-related fat deposition in multifidus muscle could be a marker for nonalcoholic fatty liver disease. J Gastroenterol. 2010;45:218–224. doi: 10.1007/s00535-009-0147-2. [DOI] [PubMed] [Google Scholar]
- 13.Kitajima Y., Hyogo H., Sumida Y., Eguchi Y., Ono N., Kuwashiro T. Severity of non-alcoholic steatohepatitis is associated with substitution of adipose tissue in skeletal muscle. J Gastroenterol Hepatol. 2013;28:1507–1514. doi: 10.1111/jgh.12227. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka M., Okada H., Hashimoto Y., Kumagai M., Nishimura H., Oda Y. Relationship between nonalcoholic fatty liver disease and muscle quality as well as quantity evaluated by computed tomography. Liver Int. 2020;40:120–130. doi: 10.1111/liv.14253. [DOI] [PubMed] [Google Scholar]
- 15.Albano D., Messina C., Vitale J., Sconfienza L.M. Imaging of sarcopenia: old evidence and new insights. Eur Radiol. 2020;30 doi: 10.1007/s00330-019-06573-2. 2199–08. [DOI] [PubMed] [Google Scholar]
- 16.Delzenne N.M., Neyrinck A.M., Cani P.D. Gut microbiota and metabolic disorders: how prebiotic can work? Br J Nutr. 2013;109:S81–S85. doi: 10.1017/S0007114512004047. [DOI] [PubMed] [Google Scholar]
- 17.Knudsen C., Neyrinck A.M., Lanthier N., Delzenne N.M. Microbiota and nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. 2019;22:393–400. doi: 10.1097/MCO.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 18.Hiel S., Gianfrancesco M.A., Rodriguez J., Portheault D., Leyrolle Q., Bindels L.B. Link between gut microbiota and health outcomes in inulin -treated obese patients: lessons from the Food4Gut multicenter randomized placebo-controlled trial. Clin Nutr. 2020;39:3618–3628. doi: 10.1016/j.clnu.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Lanthier N. Non-alcoholic steatohepatitis in 2018. Louv Med. 2018;137(5):308–313. [Google Scholar]
- 20.de Lédinghen V., Vergniol J., Foucher J., Merrouche W., Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911–918. doi: 10.1111/j.1478-3231.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 21.Myers R.P., Pomier-Layrargues G., Kirsch R., Pollett A., Duarte-Rojo A., Wong D. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012;55:199–208. doi: 10.1002/hep.24624. [DOI] [PubMed] [Google Scholar]
- 22.Lanthier N., Rodriguez J., Nachit M., Hiel S., Trefois P., Neyrinck A.M. Microbiota analysis and transient elastography reveal new extra-hepatic components of liver steatosis and fibrosis in obese patients. Sci Rep. 2021;11:659. doi: 10.1038/s41598-020-79718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heymsfield S.B., Wang Z., Baumgartner R.N., Ross R. Human body composition: advances in models and methods. Annu Rev Nutr. 1997;17:527–558. doi: 10.1146/annurev.nutr.17.1.527. [DOI] [PubMed] [Google Scholar]
- 24.Martin L., Birdsell L., MacDonald N., Reiman T., Clandinin M.T., McCargar L.J. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 25.Mourtzakis M., Prado C.M.M., Lieffers J.R., Reiman T., McCargar L.J., Baracos V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 26.Goodpaster B.H., Kelley D.E., Thaete F.L., He J., Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89:104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 27.Nachit M., Kwanten W.J., Thissen J.-P., Op De Beeck B., Van Gaal L., Vonghia L. Muscle fat content is strongly associated with NASH: a longitudinal study in patients with morbid obesity. J Hepatol. 2021 doi: 10.1016/j.jhep.2021.02.037. [DOI] [PubMed] [Google Scholar]
- 28.Vilar-Palop J., Vilar J., Hernández-Aguado I., González-Álvarez I., Lumbreras B. Updated effective doses in radiology. J Radiol Prot. 2016;36:975–990. doi: 10.1088/0952-4746/36/4/975. [DOI] [PubMed] [Google Scholar]
- 29.Gervaise A., Osemont B., Louis M., Lecocq S., Teixeira P., Blum A. Standard dose versus low-dose abdominal and pelvic CT: comparison between filtered back projection versus adaptive iterative dose reduction 3D. Diagn Interv Imaging. 2014;95:47–53. doi: 10.1016/j.diii.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 30.International Commission on Radiological Protection The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007;37(2–4):1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Cai C., Song X., Chen Y., Chen X., Yu C. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Hepatol Int. 2020;14:115–126. doi: 10.1007/s12072-019-09964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choe E., Kang H., Park B., Yang J., Kim J. The association between nonalcoholic fatty liver disease and CT-measured skeletal muscle mass. J Clin Med. 2018;7:310. doi: 10.3390/jcm7100310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng T.-C., Wu L.-W., Chen W.-L., Liaw F.-Y., Chang Y.-W., Kao T.-W. Nonalcoholic fatty liver disease and sarcopenia in a Western population (NHANES III): the importance of sarcopenia definition. Clin Nutr. 2019;38:422–428. doi: 10.1016/j.clnu.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Chen V.L., Wright A.P., Halligan B., Chen Y., Du X., Handelman S.K. Body composition and genetic lipodystrophy risk score associate with nonalcoholic fatty liver disease and liver fibrosis. Hepatol Commun. 2019;3:1073–1084. doi: 10.1002/hep4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomlinson D.J., Erskine R.M., Morse C.I., Winwood K., Onambélé-Pearson G. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology. 2016;17:467–483. doi: 10.1007/s10522-015-9626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aleixo G.F.P., Shachar S.S., Nyrop K.A., Muss H.B., Malpica L., Williams G.R. Myosteatosis and prognosis in cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol. 2020;145:102839. doi: 10.1016/j.critrevonc.2019.102839. [DOI] [PubMed] [Google Scholar]
- 37.Ebadi M., Montano-Loza A.J. Clinical relevance of skeletal muscle abnormalities in patients with cirrhosis. Dig Liver Dis. 2019;51:1493–1499. doi: 10.1016/j.dld.2019.05.034. [DOI] [PubMed] [Google Scholar]
- 38.Cava E., Yeat N.C., Mittendorfer B. Preserving healthy muscle during weight loss. Adv Nutr. 2017;8:511–519. doi: 10.3945/an.116.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eslamparast T., Montano-Loza A.J., Raman M., Tandon P. Sarcopenic obesity in cirrhosis – the confluence of 2 prognostic titans. Liver Int. 2018;38:1706–1717. doi: 10.1111/liv.13876. [DOI] [PubMed] [Google Scholar]
- 40.Nachit M., De Rudder M., Thissen J.-P., Schakman O., Bouzin C., Horsmans Y. Myosteatosis rather than sarcopenia associates with non-alcoholic steatohepatitis in non-alcoholic fatty liver disease preclinical models. J Cachexia Sarcopenia Muscle. 2021;12:144–158. doi: 10.1002/jcsm.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castera L., Friedrich-Rust M., Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156 doi: 10.1053/j.gastro.2018.12.036. 1264–1281.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yada N., Sakurai T., Minami T., Arizumi T., Takita M., Hagiwara S. Influence of liver inflammation on liver stiffness measurement in patients with autoimmune hepatitis evaluation by combinational elastography. Oncology. 2017;92:10–15. doi: 10.1159/000451011. [DOI] [PubMed] [Google Scholar]
- 43.Yoo J.-J., Seo Y.S., Kim Y.S., Jeong S.W., Jang J.Y., Suh S.J. The influence of histologic inflammation on the improvement of liver stiffness values over 1 and 3 Years. J Clin Med. 2019;8:2065. doi: 10.3390/jcm8122065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.