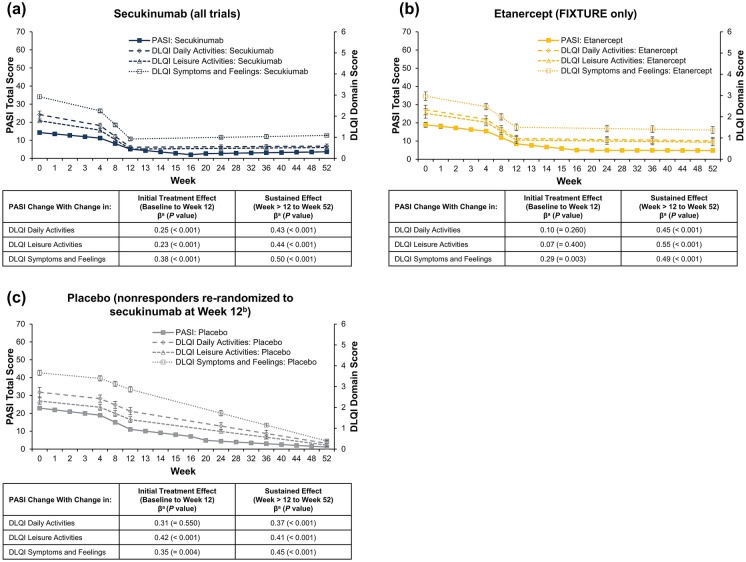
Fig. 3.
Correlation of change in PASI score with DLQI domains in patients with PsO receiving a secukinumab, b etanercept, or c placebo. DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index; PsO, psoriasis. aβ (standardized residual covariance: i.e. the correlation between the slopes not explained by the included covariates) indicates that a change of 1 standardized value on the PASI will result in a change in a standardized value on the DLQI at the reported value (e.g. 0.25 for the initial treatment exposure of secukinumab on daily activities). bPatients who did not achieve PASI75 at week 12 were re-randomized 1:1 to receive secukinumab 300–150 mg