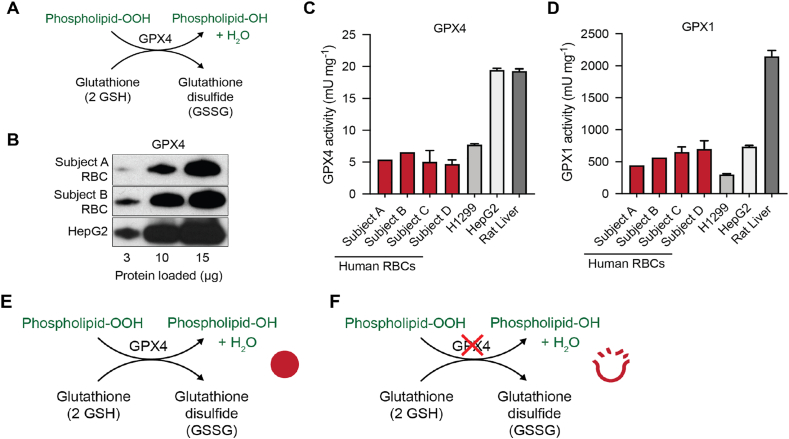
Fig. 5.
RBCs have GPx4 protein that has the classic peroxidase activity of GPx4.
(A) Schematic of the peroxidase activity of GPx4.
(B) GPx4 protein expression detected by Western blot analysis of RBCs and HepG2 cell lysates. The expected increase in intensity is observed with increasing amounts of protein loaded.
(C) RBCs have lower activity of GPx4 compared to human lung carcinoma (H1299), human hepatocellular carcinoma (HepG2) cell lines, and rat liver. Error bars (standard deviation) represent replicates of a single sample ran >3 times in our combined assay for GPx1 and GPx4 activities.
(D) GPx1 activity of RBCs was comparable to human carcinoma cell lines, but lower than rat liver. The observed activity of GPx4 is approximately 1% of that of GPx1 in each of the samples, although absolute comparison of measured activities of GPx4 and GPx1 is not possible due to different kinetics in the activity assays. Note the scales for the ordinates of panels C and D differ by a factor of 100. (See Supplementary Data for a discussion of the kinetics of the combination assay.) Error bars (standard deviation) represent replicates of a single sample ran >3 times in our combined assay for GPx1 and GPx4 activities. The subjects, A – D, in this figure are different from subjects #1 – #4 in Fig. S4.
(E) GPx4 may prevent potential oxidative damage of RBCs by removing phospholipid hydroperoxides.
(F) Reduced concentration and activity of GPx4 could lead to increased oxidative damage by the reactions of phospholipid-OOH, leading to increased rates of hemolysis during storage of RBCs.