Abstract
Several studies reported that aspirin can potentially help prevent infection and serious complications of coronavirus disease (COVID-19), but no study has elucidated a definitive association between aspirin and COVID-19. This study aims to investigate the association between aspirin and COVID-19.
This case-control study used demographic, clinical, and health screening laboratory test data collected from the National Health Insurance Service database. Patients who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection until June 4, 2020, were matched with control patients using propensity score matching according to their SARS-CoV-2 status, the composite of complications, and death. The composite of complications included intensive care unit admission, use of vasopressors, high-flow oxygen therapy, renal replacement therapy, extracorporeal membrane oxygenation, and death. Exposure to aspirin was defined as having a prescription for aspirin for more than 14 days, including the index date. After matching, multivariable-adjusted conditional logistic regression analysis was performed. To confirm the robustness of this study, we used 2 study groups, 3 propensity score matching methods, and 3 models for conditional logistic regression analyses.
The crude odds ratio and 95% confidence interval for SARS-CoV-2 infection between the groups without and with exposure to aspirin were 1.21 (1.04–1.41), but the adjusted odds ratios (95% confidence interval) were not significant. There was no association between aspirin exposure and COVID-19 status. Multiple statistical analyses, including subgroup analysis, revealed consistent results. Furthermore, the results of analysis for complications and death were not significant. Aspirin exposure was not associated with COVID-19-related complications and mortality in COVID-19 patients.
In this nationwide population-based case-control study, aspirin use was not associated with SARS-CoV-2 infection or related complications. With several ongoing randomized controlled trials of aspirin in COVID-19 patients, more studies would be able to confirm the effectiveness of aspirin in COVID-19.
Keywords: aspirin, COVID-19, infections, Republic of Korea
1. Introduction
On March 11, 2020, the World Health Organization (WHO) declared the current coronavirus disease (COVID-19) outbreak as a pandemic. As of February 5, 2021, a total of 103,989,900 cases have been confirmed worldwide, with 2,260,259 deaths, according to the WHO.[1] The commonest clinical features of COVID-19 include fever, respiratory symptoms, and myalgia; however, severe symptoms, with lung and systematic inflammation, can result in diffuse alveolar damage, multiple organ dysfunction, and mortality.[2,3] In addition, the main pathological features of COVID-19 include virus-mediated damage, primarily in the respiratory tract and multiple organs, activation of the immune response following the release of pro-inflammatory cytokines, overactivation of the coagulation cascade, and platelet aggregation that can lead to thrombosis.[4,5]
Since it was first synthesized in 1898, aspirin has been widely used to treat and prevent various human diseases, including cardiovascular diseases. As a nonselective cyclooxygenase inhibitor, aspirin can block prostaglandin and thromboxane secretion.[6] Consequently, aspirin has anti-inflammatory, analgesic, antipyretic, and antithrombotic effects.[7] Along with its effects in reducing inflammation and platelet aggregation, it has been proposed that aspirin inhibits the viral replication of DNA and RNA viruses, although the exact mechanism remains unclear.[8] Systematic inflammation and thrombotic complications commonly occur in COVID-19 patients;[9] theoretically, aspirin use might facilitate the prevention of COVID-19 and its serious complications. Thus, the need for research on the prophylactic or therapeutic effects of aspirin on COVID-19 has been highlighted.[7,10]
Chow et al[11] reported that hospitalized COVID-19 patients, who received low-dose aspirin daily for protection against cardiovascular diseases, had a significantly lower risk of complications and mortality, compared with those who did not receive this medication. On the other hand, Yuan et al[12] reported that the use of low-dose aspirin was unassociated with the clinical outcome of COVID-19 patients with coronary artery disease. The prophylactic use of aspirin is controversial and the related studies are limited. Therefore, this study aims to investigate the association between aspirin and COVID-19 using South Korea's nationwide healthcare database that comprises information on all severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive patients in the region.
2. Methods
2.1. Data source
This case-control study used data derived from the National Health Insurance Service (NHIS) database that was linked with the health screening program and the Korea Centers for Disease Control and Prevention (KCDC) databases. Generally, the NHIS database contains demographic data (age, sex, residential area, and income level), clinical data (medical visits, diagnosis, and treatment), and health screening data, including laboratory test results, between 2015 and 2018.[13,14] The KCDC database provides laboratory confirmation for individuals tested for the SARS-CoV-2 infection.[15] The detailed codes for diseases and treatments in this study are available in Supplementary Table 1.
2.2. Study population
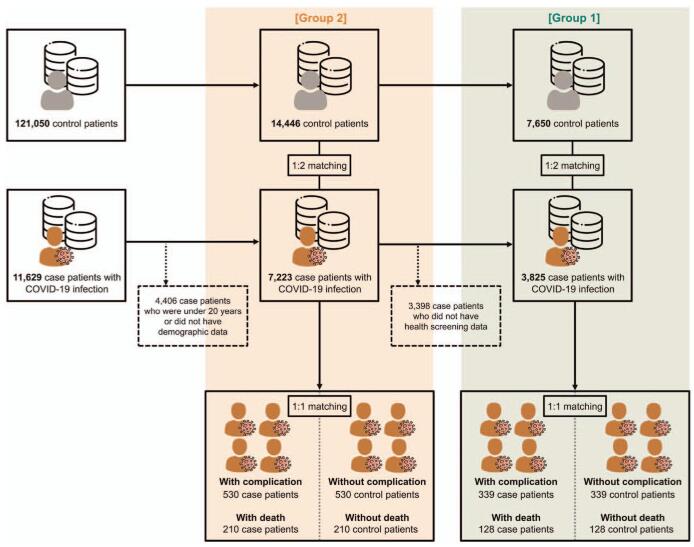
The flow of information regarding the study participants’ is described in Figure 1. The study included 11,629 individuals who were all tested positive for COVID-19 with the diagnostic codes B342, B972, U071, and U072, until June 4, 2020. The laboratory diagnosis of SARS-CoV-2 infection in Korea was based on the KCDC and WHO guidelines, which recommended polymerase chain reaction amplification of the viral E gene as a screening test and amplification of the RdRp region of the orf1b gene as a confirmatory test.[16] We excluded 4406 patients who were younger than 20 years or whose demographic data were unavailable and 3398 patients with missing health screening data. Overall, 3825 patients had no missing values (designated as group 1), whereas 7223 patients had missing values for health screening only (designated as group 2). To confirm the robustness of this study and minimize selection bias, we used these 2 groups as the study populations.
Figure 1.
The flow of the case-control study.
2.3. Study outcomes
We evaluated 3 indices (SARS-CoV-2 infection status, composite of complications, and death) to investigate the association between aspirin and COVID-19 and its related complications.[17–19] The composite of complications included intensive care unit admission; use of vasopressors, high-flow oxygen therapy, renal replacement therapy, extracorporeal membrane oxygenation, and mortality. Among patients with COVID-19, those included in the analysis for complications and death were 339 and 128 in group 1, and 530 and 210 in group 2.
2.4. Exposure to aspirin
As this study used a population-based NHIS database, the date of the SARS-CoV-2 test for individuals was set as the index date. Exposure to aspirin was defined as having a prescription for aspirin for more than 14 days, including the index date.
2.5. Covariates
With regard to the residential area, the patients were classified under Daegu and Gyeongbuk, Seoul and Gyeonggi, or other areas, due to the uneven distribution of the COVID-19 outbreak in Korea.[20] According to income levels, participants were classified into quantiles. The history of underlying diseases was determined based on the International Classification of Diseases, 10th Revision diagnosis codes. The comorbidities included diabetes, hypertension, dyslipidemia, cardiovascular disease, lung disease, liver disease, cancer, end-stage renal disease with dialysis, and immunocompromised status. The Charlson Comorbidity Index (CCI) for measuring the total comorbidity burden was calculated and patients were further grouped based on a CCI score of 0, 1, 2, and ≥3.[21]
Findings from the last health screening, which included the body mass index, systolic blood pressure, diastolic blood pressure, hemoglobin, fasting glucose, aspartic acid transaminase, alanine transaminase, gamma glutamyl transpeptidase, cholesterol, triglyceride, high-density lipoprotein cholesterol, and estimated glomerular filtration rate, for individual patients were used. Laboratory tests were performed on blood samples collected after fasting for at least 8 hours. The body mass index was calculated as the weight in kilograms divided by height in meters squared, whereas the estimated glomerular filtration rate was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation for creatinine.[22] Information about smoking status was obtained using the health screening questionnaires, and patients were categorized as nonsmoker, ex-smoker, or current smoker.
2.6. Statistical analysis
The baseline characteristics are presented as mean with standard deviation for continuous variables and number with percentage (%) for categorical variables. Comparisons between cases and controls were performed using the Student t tests for continuous variables and the χ2 or Fisher exact tests for categorical variables. To reduce the bias introduced by confounding variables and to mimic the analysis in randomized controlled trials, we used propensity score matching (PSM).[23] Furthermore, to ensure the robustness of the results of this case-control study, we used 3 PSM methods and 3 logistic regression analysis models. For groups 1 and 2, we matched the cases and controls using PSM by sex, age with 10-yearly intervals, residential area, and income level (PSM 1); further matched by underlying diseases (PSM 2); and health screening findings (PSM 3). The matching was exact by sex, and nearest-neighbor matching was performed for other variables, with a caliper of 0.1 in propensity scores. Based on SARS-CoV-2 status, case patients (positive) and control patients (negative) were matched with a ratio of 1:2. With 2 indices, including the composite of complications and death, a 1:1 case and control matching was performed in the same manner as described earlier. The standardized mean differences (SMD) between the cases and controls from before to after PSM are shown in Supplementary Figures 1–3. The SMD is most commonly used to investigate the balance in the distribution of confounding variables between the 2 groups. The SMD less than 0.1 can be considered a sign of balance.[24] Following the case-control matching, the odds ratio (OR) and 95% confidence interval (95% CI) were calculated using conditional logistic regression analysis. In cases matched by sex, age, residential area, and income level, we performed multivariable-adjusted conditional logistic regression analysis for outcomes with adjustments for the presence of diabetes, hypertension, dyslipidemia, cardiovascular disease, lung disease, liver disease, cancer, immunocompromised status, end-stage renal disease with dialysis, and CCI in groups 1 and 2 (model 1). For group 1, further adjustments were also performed for the findings from health screening data (model 2). In cases matched by PSM 2, adjustment was performed for health screening findings for group 1 (model 3). In summary, we used 2 study groups, 3 PSM methods, and 3 models in the logistic regression analysis. Furthermore, a subgroup analysis of COVID-19 patients according to sex (male and female) and age (≥50 years and <50 years) was performed to evaluate the risk stratification. Statistical analyses were performed using SAS version 9.4 software (SAS Institute Inc, Cary, NC) and a P value <.05 was considered statistically significant.
2.7. Ethical considerations
This study was approved and the requirement of informed consent was waived by the Institutional Review Board of the Gwangju Institute of Science and Technology (20200630-EX-02–02) as all of the data used in the study were anonymized for protecting the confidentiality of personal information. This study was performed in accordance with the Declaration of Helsinki, 1964 and its later amendments.
3. Results
3.1. Baseline characteristics of study population
After matching by SARS-CoV-2 status, the numbers of cases and controls were 3825 and 7650 in group 1, and 7223 and 14,446 in group 2. The baseline characteristics are presented in Table 1 and Supplementary Table 2. There was no significant intergroup difference in sex, age, residential area, and income level due to matching. Both underlying diseases and a high CCI score were more frequent in cases than in controls. Laboratory findings from health screening were significantly different between cases and controls, except for aspartic acid transaminase, alanine transaminase, total cholesterol, and HDL-C, in group 1. Moreover, the rates of complication were significantly higher in cases than in controls of both groups (P < .001).
Table 1.
Baseline characteristics according to COVID-19 (group 1).
| Characteristics | Case patients (n = 3825) | Control patients (n = 7650) | P_value |
| Sex, male, n (%) | 1405 (36.7) | 2810 (36.7) | 1.000 |
| Age, intervals, n (%) | |||
| 20–29 yrs | 193 (5.1) | 386 (5.1) | 1.000 |
| 30–39 yrs | 353 (9.2) | 706 (9.2) | |
| 40–49 yrs | 659 (17.2) | 1318 (17.2) | |
| 50–59 yrs | 1122 (29.3) | 2244 (29.3) | |
| 60–69 yrs | 871 (22.8) | 1742 (22.8) | |
| 70–79 yrs | 430 (11.2) | 860 (11.2) | |
| ≥ 80 yrs | 197 (5.2) | 394 (5.2) | |
| Residential area, n (%) | |||
| Daegu and Gyeongbuk | 2986 (78.1) | 5972 (78.1) | 1.000 |
| Income, n (%) | |||
| First quantile (lowest) | 832 (21.8) | 1664 (21.8) | 1.000 |
| Second quantile | 562 (14.7) | 1124 (14.7) | |
| Third quantile | 704 (18.4) | 1408 (18.4) | |
| Fourth quantile | 740 (19.3) | 1480 (19.3) | |
| Fifth quantile (highest) | 987 (25.8) | 1974 (25.8) | |
| Underlying disease, n (%) | |||
| Diabetes | 711 (18.6) | 1180 (15.4) | <.001 |
| Hypertension | 1458 (38.1) | 2857 (37.3) | .421 |
| Dyslipidemia | 2523 (66.0) | 5007 (65.5) | .588 |
| Cardiovascular disease | 624 (16.3) | 926 (12.1) | <.001 |
| Lung disease | 1869 (48.9) | 3207 (41.9) | <.001 |
| Liver disease | 2229 (58.3) | 3754 (49.1) | <.001 |
| Cancer | 328 (8.6) | 560 (7.3) | .018 |
| Immunocompromised status | 488 (12.8) | 818 (10.7) | .001 |
| End-stage renal disease with dialysis | 9 (0.2) | 11 (0.1) | .268 |
| Charlson Comorbidity Index | |||
| 0 | 1552 (40.6) | 3334 (43.6) | .002 |
| 1 | 959 (25.1) | 1915 (25.0) | |
| 2 | 576 (15.0) | 1122 (14.7) | |
| ≥ 3 | 738 (19.3) | 1279 (16.7) | |
| Smoking status, n (%) | |||
| Non-smoker | 2971 (77.7) | 5491 (71.8) | <.001 |
| Ex-smoker | 577 (15.1) | 1023 (13.4) | |
| Current smoker | 277 (7.2) | 1136 (14.8) | |
| Health screening finding | |||
| BMI, mean (SD), kg/m2 | 24.0 (3.4) | 22.8 (3.4) | <.001 |
| SBP, mean (SD), mm Hg | 121.5 (15.2) | 122.4 (15.1) | .003 |
| DBP, mean (SD), mm Hg | 74.9 (10.0) | 75.3 (9.8) | .04 |
| Hemoglobin, mean (SD), g/dL | 13.7 (1.6) | 13.8 (1.6) | .002 |
| Fasting glucose, mean (SD), mg/dL | 101.1 (28.0) | 99.5 (22.7) | .001 |
| AST, mean (SD), U/L | 25.3 (20.2) | 25.6 (18.9) | .431 |
| ALT, mean (SD), U/L | 24.3 (29.1) | 24.2 (25.6) | .850 |
| GGT, mean (SD), U/L | 29.9 (36.1) | 32.6 (47.7) | .002 |
| Total cholesterol, mean (SD), mg/dL | 195.4 (38.1) | 196.4 (38.5) | .186 |
| Triglyceride, mean (SD), mg/dL | 121.5 (85.3) | 126.4 (93.7) | .006 |
| HDL-C, mean (SD), mg/dL | 58.0 (30.3) | 58.4 (23.5) | .434 |
| eGFR, mean (SD), mL/min/1.73m2 | 90.3 (22.4) | 91.3 (23.6) | .029 |
| Complication, n (%) | 346 (9.1) | 84 (1.1) | <.001 |
| Death | 128 (3.3) | 11 (0.1) | <.001 |
| Intensive care unit admission | 175 (4.6) | 9 (0.1) | <.001 |
| Vasopressor use | 195 (5.1) | 68 (0.9) | <.001 |
| High flow oxygen therapy | 194 (5.1) | 2 (0.1) | <.001 |
| Renal replacement therapy | 34 (0.9) | 8 (0.1) | <.001 |
| Extracorporeal membrane oxygenation | 34 (0.9) | 8 (0.1) | <.001 |
After matching for complications and mortality, the baseline characteristics are presented in Supplementary Tables 3 and 4. Overall, there were no significant differences in sex, age, residential area, and income level between cases and controls. However, the cases had a higher number of underlying diseases and a higher CCI score than the control patients.
3.2. Association between exposure to aspirin and COVID-19
The results of the logistic regression analysis of COVID-19 are shown in Table 2. In group 1, the crude OR (95% CI) for COVID-19 between the non-exposure and exposure to aspirin groups was 1.21 (1.04–1.41). After adjustment of models 1 and 2, the ORs (95% CI) were not significant (1.08 [0.92–1.27] and 1.11 [0.94–1.30]). Furthermore, this trend was observed when the PSM 2 and 3 methods were applied. In group 2, the crude OR (95% CI) for COVID-19 was significant, but the adjusted OR (95% CI) was not significant. Following the implementation of the PSM2 methods in the logistic regression analysis, the adjusted OR (95% CI) was insignificant.
Table 2.
Association between exposure to aspirin and COVID-19 (groups 1 and 2).
| Case patients (%) | Control patients (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Group 1 | |||||
| PSM 1 | 3825 (100) | 7650 (100) | Model 1∗ | Model 2† | |
| Non-exposure to aspirin | 3512 (91.8) | 7119 (93.1) | 1.00 | 1.00 | 1.00 |
| Exposure to aspirin | 313 (8.2) | 531 (6.9) | 1.21 (1.04–1.41) | 1.08 (0.92–1.27) | 1.11 (0.94–1.30) |
| PSM 2 | 3825 (100) | 7650 (100) | Model 3‡ | ||
| Non-exposure to aspirin | 3512 (91.8) | 7033 (91.9) | 1.00 | 1.00 | |
| Exposure to aspirin | 313 (8.2) | 617 (8.1) | 1.20 (0.87–1.20) | 1.02 (0.87–1.21) | |
| PSM 3 | 128 (100) | 128 (100) | |||
| Non-exposure to aspirin | 3512 (91.8) | 7042 (92.1) | 1.00 | ||
| Exposure to aspirin | 313 (8.2) | 608 (7.9) | 1.03 (0.90–1.19) |
| Group 2 | |||||
| PSM 1 | 7223 (100) | 14,446 (100) | Model 1∗ | ||
| Non-exposure to aspirin | 6792 (94.0) | 13,694 (94.8) | 1.00 | 1.00 | |
| Exposure to aspirin | 431 (6.0) | 752 (5.2) | 1.18 (1.03–1.34) | 1.01 (0.88–1.16) | |
| PSM 2 | 7222 (100) | 14,443 (100) | |||
| Non-exposure to aspirin | 6792 (94.1) | 13,568 (93.9) | 1.00 | ||
| Exposure to aspirin | 430 (5.9) | 875 (6.1) | 0.98 (0.85–1.12) |
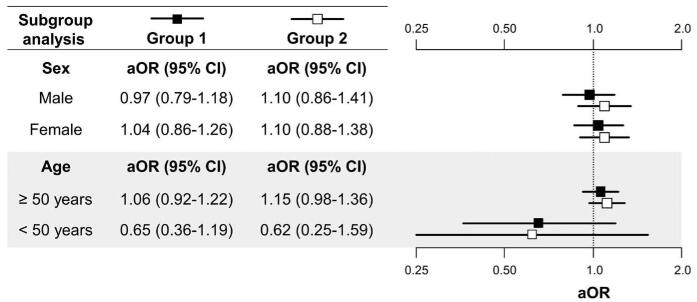
3.3. Subgroup analysis according to sex and age for aspirin and COVID-19
Using the PSM 1 method, subgroup analysis by sex and age was performed for the risk stratification, and the results are shown in Figure 2. All analyses for aspirin and COVID-19 were not significant, regardless of sex or age.
Figure 2.
Subgroup analysis according to sex and age (groups 1 and 2). aOR = adjusted odds ratio, CI = confidence interval. Group 1, adjusted for comorbidities, the Charlson Comorbidity Index, and health-screening findings. Group 2, adjusted for comorbidities and the Charlson Comorbidity Index.
3.4. Association between exposure to aspirin and COVID-19-related complications
The results of the logistic regression analysis for complications and death are shown in Table 3. In groups 1 and 2, the crude and adjusted ORs (95% CI) for complications and death were not significant.
Table 3.
Association between exposure to aspirin and COVID-19 related complications (groups 1 and 2).
| Case patients (%) | Control patients (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Group 1 | |||||
| Complication | 339 (100) | 339 (100) | Model 1∗ | Model 2† | |
| Non-exposure to aspirin | 262 (77.3) | 281 (82.9) | 1.00 | 1.00 | 1.00 |
| Exposure to aspirin | 77 (22.7) | 58 (17.1) | 1.48 (0.99–2.20) | 1.06 (0.66–1.69) | 1.07 (0.65–1.75) |
| Death | 128 (100) | 128 (100) | |||
| Non-exposure to aspirin | 91 (71.1) | 97 (75.8) | 1.00 | 1.00 | 1.00 |
| Exposure to aspirin | 37 (28.9) | 31 (24.2) | 1.26 (0.73–2.18) | 0.92 (0.46–1.84) | 0.76 (0.34–1.71) |
| Group 2 | |||||
| Complication | 530 (100) | 530 (100) | Model 1∗ | ||
| Nonexposure to aspirin | 418 (78.9) | 433 (81.7) | 1.00 | 1.00 | – |
| Exposure to aspirin | 112 (21.1) | 97 (18.3) | 1.21 (0.88–1.64) | 0.91 (0.64–1.29) | – |
| Death | 210 (100) | 210 (100) | |||
| Non-exposure to aspirin | 152 (72.4) | 156 (74.3) | 1.00 | 1.00 | – |
| Exposure to aspirin | 58 (27.6) | 54 (25.7) | 1.10 (0.72–1.66) | 0.89 (0.53–1.47) | – |
4. Discussion
Aspirin has both anti-inflammatory and antithrombotic effects that are produced via the inhibition of prostaglandin and thromboxane synthesis by the irreversible inactivation of cyclo-oxygenase.[6,7] Furthermore, aspirin may inhibit virus replication by suppressing prostaglandin E2 in the macrophages and by upregulating type I interferon production.[25] Consequently, aspirin can stimulate immune activity against viral infection, and this effect has been used against a variety of viruses.[26] Therefore, aspirin, with its 3 effects, was proposed as a reasonable therapeutic or prophylactic candidate in COVID-19.[7] However, virus-mediated injury to multiple organs, systematic inflammation, and platelet hyperaggregability are effects similar to those seen in the systemic inflammatory response syndrome and sepsis.[27,28] Earlier studies that investigated the effect of aspirin on sepsis susceptibility and outcomes have drawn various conclusions. For instance, in a nested cohort study, Al Harbi et al[29] found that aspirin use in intensive care unit patients was associated with increased rates of severe sepsis. In addition, Wiewel et al[30] performed a prospective cohort study with 972 intensive care unit patients and reported that prior antiplatelet therapy was not associated with the severity and mortality of sepsis. Hsu et al[31] reported that the baseline aspirin use was not associated with rates of sepsis in a prospective cohort study. From the AspiriN To Inhibit SEPSIS randomized controlled trial, daily aspirin treatment did not reduce deaths due to sepsis in community-dwelling older adults.[32] Our findings were consistent with the indirect evidence from these previous sepsis-related studies.
The exact mechanism that provides an explanation of these results has not been elucidated, but several possible rationales can be suggested. First, aspirin could induce various deleterious effects in critically ill patients. As an antithrombotic drug, aspirin depresses platelet inhibition through the interaction of inflammatory mediators with immune cells and modulates the adverse effects associated with inflammatory reactions.[33] Moreover, aspirin stimulates the synthesis of nitric oxide, which inhibits the interactions between leukocytes and endothelial cells, and decreases neutrophil recruitment.[34] In addition, aspirin inhibits the secretion of nuclear factor kappa-B, which is important in immune and inflammatory responses.[35] The lack of an advantageous effect of aspirin on COVID-19 and its complications may be a reflection of the interaction of these various pathways. Second, the use of nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, in COVID-19 patients is a controversial issue. Previous reports suggested a possibly increased risk of poor outcomes with NSAID use in COVID-19 patients.[19,36] Furthermore, experimental data showed that NSAIDs can alter the intrinsic functions of neutrophils and negatively affect the inflammatory pathways.[37] From this perspective, aspirin neutralizes its harmful effects to some extent due to the additional effects of inhibiting thrombosis and viral replication, compared with other NSAIDs.
Chow et al[11] reported that hospitalized COVID-19 patients on daily low-dose aspirin therapy had a significantly better prognosis than patients who did not receive daily low-dose aspirin. However, the abovementioned study focused on a limited number of COVID-19 inpatients, among whom approximately 30% to 50% had complications. There were no significant differences in overt thrombosis events between aspirin users and nonusers despite their COVID-19 status. In comparison with that study, our study has several strengths. In this case-control study, we used data (demographic, clinical, and health-screening information) from the NHIS database, which comprises information from a sufficiently large population and multiple variables to evaluate clinically important issues. The NHIS is a universal single-paying healthcare provider that covers all insurance claims and, therefore, the database contained information on both inpatients and outpatients.[13,14] In addition, research using claims data has the advantage of being able to proceed quickly even in the restricted environment due to the COVID-19 pandemic. Our results were consistent with the results of various statistical analyses from 2 different study groups, indicating that there was no association between aspirin exposure and COVID-19 and the related complications.
To minimize the risk of complications and mortality with COVID-19, several drugs have been repurposed for use in COVID-19 treatment.[38,39] Determining the usefulness of the available drugs in COVID-19 treatment is advantageous because these drugs could be promptly evaluated in large-scale studies to provide an opportunity to reduce the risk of complications and death from COVID-19.[40] Accordingly, there are at least 7 ongoing randomized controlled trials (NCT04445623, NCT04410328, NCT04365309, NCT04324463, NCT04381936, NCT04333407, and NCT04703608) that are investigating the use of aspirin or antiplatelet drugs to mitigate of the risk of complications and deaths in patients with COVID-19. Along with the results of our study, these ongoing studies can clarify the potential benefits and risks of the specified drugs in COVID-19 patients.
However, there are limitations of this study that should be acknowledged. First, there could have been discrepancies between the actual therapeutic practices and those reported in insurance claims. To ensure the robustness of results in this study, we used widely accepted definitions of the clinical outcomes as well as covariates from previous studies.[17–19] Second, the date of exposure to aspirin could have been misclassified. Accordingly, we used various statistical methods, and our findings were consistent from before to after the adjustment. Third, despite the implementation of multiple statistical methods, we could not eliminate the residual confounding by unmeasurable factors, which is an inherent limitation of observational studies.
In this nationwide population-based case-control study, exposure to aspirin was unassociated with COVID-19 or its related complications. As several randomized controlled trials of aspirin in COVID-19 patients are ongoing, findings from more studies of aspirin and COVID-19 could possibly confirm the effectiveness of aspirin.
Acknowledgments
The authors thank Korean National Health Insurance Service and Korea Centers for Disease Control and Prevention for providing access to their data (NHIS-2020-1-483).
Author contributions
Conceptualization: Minkook Son, Myung-giun Noh, Jeongkuk Seo, Hansoo Park, Sung Yang.
Data curation: Minkook Son.
Formal analysis: Minkook Son, Myung-giun Noh, Jeong Hoon Lee.
Funding acquisition: Hansoo Park, Sung Yang.
Investigation: Minkook Son, Myung-giun Noh.
Methodology: Minkook Son, Myung-giun Noh, Jeongkuk Seo, Hansoo Park, Sung Yang.
Project administration: Minkook Son, Sung Yang.
Software: Minkook Son.
Supervision: Hansoo Park, Sung Yang.
Visualization: Minkook Son, Myung-giun Noh, Jeong Hoon Lee.
Writing – original draft: Minkook Son, Myung-giun Noh, Jeong Hoon Lee.
Writing – review & editing: Minkook Son, Myung-giun Noh, Jeongkuk Seo, Hansoo Park, Sung Yang.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CCI = Charlson Comorbidity Index, CI = confidence interval, COVID-19 = coronavirus disease, KCDC = Korea Centers for Disease Control and Prevention, NHIS = National Health Insurance Service, NSAIDs = nonsteroidal anti-inflammatory drugs, OR = odds ratio, PSM = propensity score matching, SMD = standardized mean difference, WHO = World Health Organization.
How to cite this article: Son M, Noh Mg, Lee JH, Seo J, Park H, Yang S. Effect of aspirin on coronavirus disease 2019: a nationwide case-control study in South Korea. Medicine. 2021;100:30(e26670).
MS and MN contributed equally to this work.
This work was supported by the National Research Foundation of Korea (NRF) grant, funded by the Korea government (MSIT) (No. 2020R1A5A8018367 and 2021R1A2C3008169). The funders had no role in the study design, data collection and analysis, decision for publication, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
The data that support the findings of this study are available from Korean National Health Insurance Service and Korea Centers for Disease Control and Prevention, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Korean National Health Insurance Service and Korea Centers for Disease Control and Prevention.
Supplemental digital content is available for this article.
ALT = alanine transaminase, AST = aspartic acid transaminase, BMI = body mass index, DBP = diastolic blood pressure, eGFR = estimated glomerular filtration rate, GGT = gamma glutamyl transpeptidase, HDL-C = high-density lipoprotein cholesterol, SBP = systolic blood pressure, SD = standard deviation.
CI = confidence interval, OR = odds ratio, PSM = propensity score matching.
PSM 1, cases, and controls were matched using PSM by sex, age with 10-yearly intervals, residential area, and income level. PSM 2, cases and controls were matched using PSM by sex, age with 10-yearly intervals, residential area, income level, and underlying diseases. PSM 3, cases, and controls were matched using PSM by sex, age with 10-yearly intervals, residential area, income level, underlying diseases, and health-screening findings.
Model 1, adjusted for comorbidities, and Charlson comorbidity index.
Model 2, adjusted for comorbidities, Charlson comorbidity index, and health screening findings.
Model 3, adjusted for health screening findings.
CI = confidence interval, OR = odds ratio.
Model 1, adjusted for comorbidities, and Charlson comorbidity index.
Model 2, adjusted for comorbidities, Charlson comorbidity index, and health screening findings.
References
- [1].WHO Coronavirus Disease (COVID-19) Dashboard, Available at: https://covid19.who.int/. (accessed February 5, 2021). [Google Scholar]
- [2].Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020;26:1017–32. [DOI] [PubMed] [Google Scholar]
- [3].Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol 2020;95:834–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol 2020;127:104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol 2011;72:619–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bianconi V, Violi F, Fallarino F, Pignatelli P, Sahebkar A, Pirro M. Is Acetylsalicylic acid a safe and potentially useful choice for adult patients with COVID-19 ? Drugs 2020;80:1383–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Glatthaar-Saalmüller B, Mair KH, Saalmüller A. Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study. Influenza Other Respir Viruses 2017;11:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pamukcu B. Inflammation and thrombosis in patients with COVID-19: a prothrombotic and inflammatory disease caused by SARS coronavirus-2. Anatol J Cardiol 2020;24:224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mohamed-Hussein AAR, Aly KME, Ibrahim M-EAA. Should aspirin be used for prophylaxis of COVID-19-induced coagulopathy? Med Hypotheses 2020;144:109975–109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chow JH, Khanna AK, Kethireddy S, et al. Aspirin use is associated with decreased mechanical ventilation, ICU admission, and in-hospital mortality in hospitalized patients with COVID-19. Anesth Analg 2021;132:930–41. [DOI] [PubMed] [Google Scholar]
- [12].Yuan S, Chen P, Li H, Chen C, Wang F, Wang DW. Mortality and pre-hospitalization use of low-dose aspirin in COVID-19 patients with coronary artery disease. J Cell Mol Med 2020;25:1263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan 2009;24:63–71. [DOI] [PubMed] [Google Scholar]
- [14].Song SO, Jung CH, Song YD, et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J 2014;38:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Park S, Choi GJ, Ko H. Information technology-based tracing strategy in response to COVID-19 in South Korea-Privacy Controversies. JAMA 2020;323:2129–30. [DOI] [PubMed] [Google Scholar]
- [16].Hong KH, Lee SW, Kim TS, et al. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med 2020;40:351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jung SY, Choi JC, You SH, Kim WY. Association of renin-angiotensin-aldosterone system inhibitors with COVID-19-related outcomes in Korea: a nationwide population-based cohort study. Clin Infect Dis 2020;71:2121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Son M, Seo J, Yang S. Association between renin-angiotensin-aldosterone system inhibitors and COVID-19 infection in South Korea. Hypertension 2020;76:742–9. [DOI] [PubMed] [Google Scholar]
- [19].Jeong HE, Lee H, Shin HJ, Choe YJ, Filion KB, Shin JY. Association between NSAIDs use and adverse clinical outcomes among adults hospitalized with COVID-19 in South Korea: a nationwide study. Clin Infect Dis 2020;Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shim E, Tariq A, Choi W, Lee Y, Chowell G. Transmission potential and severity of COVID-19 in South Korea. Int J Infect Dis 2020;93:339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- [22].Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–70. [DOI] [PubMed] [Google Scholar]
- [23].Austin PC. An Introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang Z, Kim HJ, Lonjon G, Zhu Y. written on behalf of AMEB-DCTCG. Balance diagnostics after propensity score matching. Ann Transl Med 2019;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Coulombe F, Jaworska J, Verway M, et al. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity 2014;40:554–68. [DOI] [PubMed] [Google Scholar]
- [26].Rajasagi NK, Bhela S, Varanasi SK, Rouse BT. Frontline Science: Aspirin-triggered resolvin D1 controls herpes simplex virus-induced corneal immunopathology. J Leukoc Biol 2017;102:1159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020;324:782–93. [DOI] [PubMed] [Google Scholar]
- [28].Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med 2020;46:854–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Al Harbi SA, Tamim HM, Al-Dorzi HM, Sadat M, Arabi YM. Association between aspirin therapy and the outcome in critically ill patients: a nested cohort study. BMC Pharmacol Toxicol 2016;17:05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wiewel MA, de Stoppelaar SF, van Vught LA, et al. Chronic antiplatelet therapy is not associated with alterations in the presentation, outcome, or host response biomarkers during sepsis: a propensity-matched analysis. Intensive Care Med 2016;42:352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hsu J, Donnelly JP, Chaudhary NS, et al. Aspirin use and long-term rates of sepsis:a population-based cohort study. PLoS One 2018;13:e0194829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Eisen DP, Leder K, Woods RL, et al. Effect of aspirin on deaths associated with sepsis in healthy older people (ANTISEPSIS): a randomised, double-blind, placebo-controlled primary prevention trial. Lancet Respir Med 2021;9:186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Evangelista V, Manarini S, Dell’Elba G, et al. Clopidogrel inhibits platelet-leukocyte adhesion and platelet-dependent leukocyte activation. Thromb Haemost 2005;94:568–77. [PubMed] [Google Scholar]
- [34].Morris T, Stables M, Hobbs A, et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol 2009;183:2089–96. [DOI] [PubMed] [Google Scholar]
- [35].Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature 1998;396:77–80. [DOI] [PubMed] [Google Scholar]
- [36].Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ 2020;368:m1086. [DOI] [PubMed] [Google Scholar]
- [37].Voiriot G, Chalumeau M, Messika J, et al. [Risks associated with the use of non-steroidal anti-inflammatory drugs during pneumonia]. Rev Mal Respir 2018;35:430–40. [DOI] [PubMed] [Google Scholar]
- [38].Singh TU, Parida S, Lingaraju MC, Kesavan M, Kumar D, Singh RK. Drug repurposing approach to fight COVID-19. Pharmacol Rep 2020;72:1479–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sultana J, Crisafulli S, Gabbay F, Lynn E, Shakir S, Trifiro G. Challenges for drug repurposing in the COVID-19 pandemic era. Front Pharmacol 2020;11:588654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dotolo S, Marabotti A, Facchiano A, Tagliaferri R. A review on drug repurposing applicable to COVID-19. Brief Bioinform 2021;22:726–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.