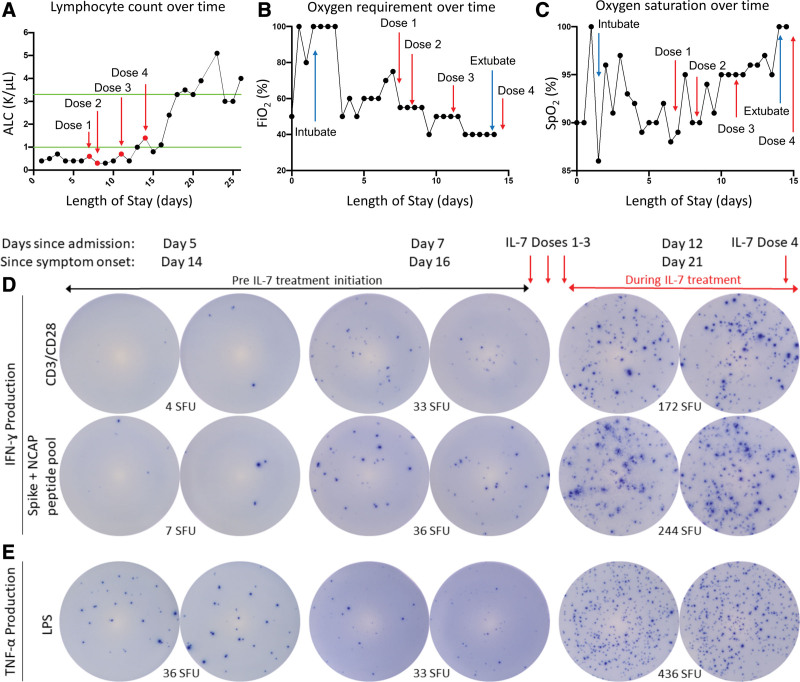
Figure 1.
Effect of interleukin (IL)–7 on clinical and immunologic variables in critically ill coronavirus disease 2019 patient. A, A test dose of IL-7 (3 μg/kg) was administered followed by three additional doses of 10 μg/kg as indicated by red arrows. IL-7 increased the absolute lymphocyte count (ALC) from ~400 lymphocytes/μL to over 5,000 lymphocytes/μL. Green lines indicate upper and lower limit of normal for ALC; IL-7 was well tolerated and did not show any evidence of worsening lung inflammation as indicated by observing a decreasing Fio2 (B) and improving oxygen saturation (C). Blue arrows in (B) and (C) indicate day of intubation and extubation. Immunologic function of patient immune effector cells was tested by enzyme-linked immunospot (ELISpot). T-cell interferon (IFN)–γ production was tested in peripheral blood mononuclear cells (PBMCs) stimulated with anti-CD3/CD28 before and after IL-7 therapy administration to the patient. 2.5 × 104 PBMCs are plated for T-cell functional assay. Note the dramatic increase in the number of lymphocytes that produce IFN-γ (as represented by number of spot forming units [SFUs]) in upper row after IL-7 therapy (D, top row); severe acute respiratory syndrome coronavirus 2 spike, and nucleocapsid peptide antigens were incubated overnight in patient PBMCs before and after IL-7 therapy. 1 × 105 PBMCs are plated to assess antigen-specific T cells. Note the dramatic increase in the number of IFN-γ–producing lymphocytes (SFU) reacting specifically to the viral antigens after IL-7 (D, second row); innate immune function was tested by ELISpot assay for tumor necrosis factor (TNF)–α in PBMCs stimulated with lipopolysaccharide (LPS). 2.5 × 103 PBMCs are plated to assess innate immune function. Note the marked increase in the number of TNF-α–producing cells (SFU) after IL-7 (E). CD = cluster of differentiation, Spo2 = oxygen saturation, NCAP = nucleocapsid protein.