Figure 1.
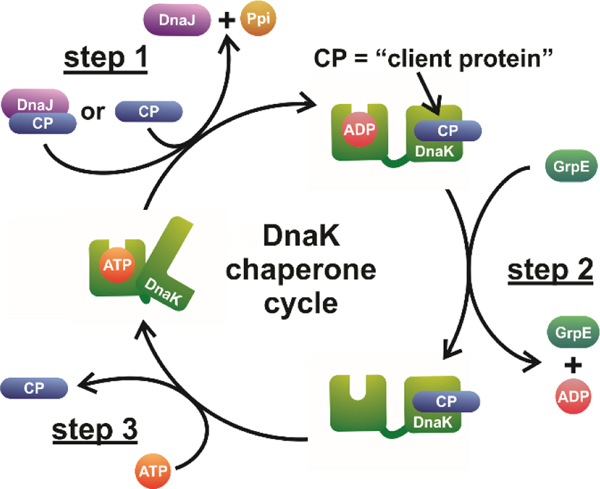
The DnaK chaperone cycle supports the proper folding of client proteins. The cycle involves interactions between unfolded, partially folded or misfolded client proteins (CP) and DnaK. These interactions are mediated by DnaK conformational changes that are allosterically regulated by nucleotide cofactors and the DnaK co-chaperones DnaJ and GrpE. The DnaK chaperone cycle can be summarized as consisting of three main steps: CP binding, nucleotide recycling and CP release. Once the client protein is released, DnaK is primed to accept new CPs for the next round of the chaperone cycle. While conformational heterogeneity of both CP and DnaK has been reported for each state of DnaK illustrated above, only the canonical conformation of each state is shown here, for simplicity.