Abstract
Background
In haemodialysis, maintaining patency of the extracorporeal circuit requires the use of anticoagulants. Although (low molecular weight) heparins are the mainstay, these are not well tolerated in all patients. Alternative approaches include saline infusion, citrate-containing dialysate, regional citrate anticoagulation or the use of heparin-coated membranes. Asymmetric cellulose triacetate (ATA) dialysers have a low degree of platelet contact activation and might be an alternative to heparin-coated dialysers. The aim of this study was to test the clotting propensity of ATA when used without systemic anticoagulation.
Methods
We performed a Phase II pilot study in maintenance dialysis patients. The ‘Strategies for Asymmetrical Triacetate dialyzer heparin-Free Effective hemodialysis’ (SAFE) study was a two-arm open-label crossover study. In Arm A, patients were dialysed using 1.9 m2 ATA membranes in combination with a citrate-containing dialysate (1 mM). In Arm B, the ATA membrane was combined with high-volume predilution haemodiafiltration (HDF) without any other anticoagulation. The primary endpoint was the success rate to complete 4 h of haemodialysis without preterm clotting. Secondary endpoints included time to clotting and measures of dialysis adequacy.
Results
We scheduled 240 dialysis sessions (120/arm) in 20 patients. Patients were randomized 1:1 to start with Arm A or B. All patients crossed to the other arm halfway through the study. A total of 232 (96.7%) study treatments were delivered. Overall, 23 clotting events occurred, 7 in Arm A and 16 in Arm B. The success rate in Arm A (ATA + citrate-containing dialysate) was 90.8/94.0% [intention to treat (ITT)/as treated]. The success rate in Arm B (ATA + predilution HDF) was 83.3/86.2% (ITT/as treated). Time to clotting was borderline significantly better in Arm A (Mantel-Cox log rank P = 0.05).
Conclusion
ATA dialysers have a low clotting propensity and both predilution HDF and a citrate-containing dialysate resulted in high rates of completed dialysis sessions.
Keywords: anticoagulation, artificial membrane, haemodialysis, heparin-grafted membrane, regional citrate anticoagulation
INTRODUCTION
Patency of the extracorporeal circuit is essential for all haemodialysis procedures. The optimal anticoagulant regimen minimizes or even completely prevents clotting of the extracorporeal circuit, has no side effects and comes at a reasonable cost [1, 2]. Unfractionated heparin (UFH) fulfils most criteria, as patency is maintained in the vast majority of dialysis sessions and it is inexpensive. In several countries, UFH has gradually been replaced by low molecular weight heparins (LMWHs). LMWHs are easy to use, as they are administered as a bolus injection and reduce membrane fibrin and platelet deposition [3, 4]. Although both UFH and LMWH provide sufficient anticoagulation, these are not free of side effects. Repetitive systemic heparinization contributes to high bleeding risk in haemodialysis patients [5, 6], especially when combined with other anticoagulants [7].
Alternative strategies have been developed to maintain the patency of the haemodialysis circuit without systemic administration of LMWH. Broadly, these can be divided into two different strategies. The first strategy aims to lower the clotting propensity of the blood. This can be achieved by dilution of the blood by bolus injections or continuous infusion of saline [predilution haemodiafiltration (HDF)], the use of short-acting anticoagulants or the use of citrate as a coagulation-limiting agent. The second strategy is based on minimization of contact activation by components of the extracorporeal circuit, mostly focused on dialyser membrane designs, e.g. heparin-grafted membranes.
Over the last decade, several different trials have sought to clarify which approach would be best practice in case heparins should be avoided. A number of conclusions can be drawn from these studies. Saline infusion in combination with a conventional membrane is less effective than the use of a heparin-grafted membrane [2, 8, 9]. Regional citrate anticoagulation is highly efficient when citrate is infused in the afferent blood line [10, 11]. Efficacy is less when citrate is added to the dialysate instead [12, 13]. More recently, clinicians started to combine anticoagulation strategies. We recently studied the combination of a heparin-coated membrane and citrate-containing dialysate, with a success rate, defined as completion of a 4-h dialysis session, of 94% [1].
While most studies suggest that heparin-coated membranes are associated with a low rate of clotting [8, 9], these are not ubiquitously available. Although regional citrate anticoagulation results in very low rates of clotting, it requires monitoring of electrolyte disturbances [10, 14]. The quest for an easy-to-perform, safe and effective systemic heparin-free anticoagulation regimen is ongoing.
Asymmetric cellulose triacetate (ATA) is a newly developed dialyser membrane with an asymmetrical pore structure [15]. By design, contact activation leading to clotting is limited. Preclinical studies confirm the low clotting propensity of ATA [15] and clinical data show the low clotting propensity of ATA, even at low doses of heparin [16]. We hypothesized that it is possible to completely abolish the use of systemic anticoagulation. We performed a Phase II exploratory study to test the clotting propensity of an ATA dialyser membrane in two prototypical modes of systemic heparin-free haemodialysis.
MATERIALS AND METHODS
Study design
This was a prospective, Phase II, open-label, two-arm, crossover study. Patients were randomized by the envelope method to start one of two study arms and were crossed to the other study arm after six haemodialysis sessions. This design allowed for an even distribution of patient characteristics, in particular related to clotting propensity.
In Arm A, patients received maintenance haemodialysis with an ATA dialyser and a citrate-containing dialysate. In Arm B, patients received maintenance predilution HDF with an ATA dialyser without systemic anticoagulation. In total, each patient was scheduled for 12 dialysis sessions (6 per arm). Patients were randomized 1:1 to start in Arm A or in Arm B.
The primary endpoint was the feasibility to maintain clinical patency of the haemodialysis extracorporeal circuit to deliver prescribed fixed-duration (4 h) haemodialysis sessions without severe clotting. This was defined as clotting of the dialyser or venous air chamber without the possibility to perform rinse back and replace the circuit or preemptive rinse back for impeding clotting. Exploratory secondary endpoints include biochemical endpoints and secondary clotting endpoints. Estimations of the degree of clotting in the venous air chamber were based on visual inspection and scores of no visible clotting (0), minimal clot formation (1), semi-occlusive clot (2) and complete clotting of the air chamber and dialyser without possibility to continue dialysis (3). Biochemical endpoints include the midweek dialysis adequacy measured as a single-pool Kt/V and the reduction ratio of β2-microglobulin, myoglobulin, p-cresol sulphate and indoxyl sulphate. Secondary clotting endpoints include the semi-quantitative clotting score (as described previously [10, 14]) and a time-to-clot analysis.
Patients
Patients ≥18 years of age on maintenance (>3 months of haemodialysis) dialysis with a ‘standard’ regimen (three dialysis sessions per week, dialysis duration 4 h) and treated in the nephrology unit of the University Hospitals Leuven (Belgium) were considered to be eligible to participate after providing signed and dated informed consent according to good clinical practice guidelines. Exclusion criteria were any known medical disorder favouring either bleeding or clotting [e.g. atypical haemolytic uraemic syndrome, antiphospholipid syndrome, idiopathic thrombocytopaenic purpura, paroxysmal nocturnal haemoglobinuria, treatment with a vitamin K antagonist and treatment with any of the non-vitamin K oral anticoagulants (apixaban, rivaroxaban, edoxaban and dabigatran)], a high risk of bleeding according to the criteria of Swartz [17], patients with a known allergic reaction to ATA or pregnancy.
Dialysis procedures
In Arm A, patients received maintenance haemodialysis with an ATA dialyser (Solacea-19H, Nipro, Osaka, Japan). Patients were maintained on their habitual blood flow (typically 300–320 mL/min). Priming volume was fixed at 1500 mL. Care was taken to remove as much air as possible from the extracorporeal circuit during priming. Dialysate flow was set at 700 mL/min during the dialysis procedure. We used a citrate-containing dialysate (Selectbag citrate, Baxter, Lessines, Belgium), resulting in a dialysate concentration of citrate 1.0 mM, calcium 1.5 mmol/L and magnesium 0.5 mmol/L. Sodium and potassium concentrations were set according to the discretion of the treating physician.
In Arm B, patients received maintenance predilution HDF with an ATA dialyser (Solacea-19H). Patients were maintained on their habitual blood flow (typically 300–320 mL/min). We aimed for a ratio between substitution volume and blood flow rate ≥80% (Qi/Qb ≥ 0.8). We used a conventional bicarbonate-based dialysate containing calcium 1.25 mmol/L and magnesium 0.5 mmol/L. Sodium and potassium concentrations were set according to the discretion of the treating physician.
The prescribed dialysis duration was fixed at 4 h. Access for dialysis was either via arteriovenous (AV) fistula or tunnelled central catheter. Only patients with an AV fistula cannulated with two needles or double-lumen tunnelled catheters were included and patients on single-needle haemodialysis were excluded from study participation. During the study period, the use of heparin or LMWH was not allowed.
Per definition, dropout from the study could be due to transfer to a different dialysis unit, transplantation, withdrawal of consent or medical reasons at the discretion of the treating physician. We also included a stopping rule. If patients had preterm interruption of the dialysis session due to clotting for three consecutive sessions, then the patient was excluded from further dialysis sessions in the study arm he/she was in. The reason for dropout was recorded in the medical records.
Biochemical analyses
Blood was sampled during the midweek dialysis session. Samples for measurement of platelets, ionized calcium and magnesium were taken at the beginning, after 30 min and 2 h and at the end of the treatment. Samples for all other measurements were taken at the start and end of the dialysis session. Haemoglobin, platelets, urea, total and ionized calcium, magnesium and β2-microglobulin were measured by the central laboratory of the University Hospitals Leuven according to routine clinical practice. Samples for measurement of myoglobin were collected and stored at −80°C for batch analysis. Samples were measured in batch by the central laboratory of the University Hospitals Leuven. Samples for indoxyl sulphate, p-cresyl sulphate and related uraemic retention solutes were collected and stored at −80°C. Samples were measured in batch as previously described [18].
Measures of dialysis adequacy included the single-pool Kt/V [19] as well as reduction ratios of β2-microglobulin, myoglobin, p-cresol sulphate and indoxyl sulphate. Reduction ratios were calculated as (concentration at start − concentration at end)/(concentration at start).
Statistical analyses
Baseline demographics are reported as mean [standard deviation (SD)] or median (minimum–maximum), depending on the distribution analysis. The primary endpoint was analysed in a two-tier approach. First, each arm was analysed separately. Success at the session level was defined as completion of a fixed-duration 4-h dialysis session without clotting necessitating preterm interruption of the dialysis session. A success of 80% at the session level and at the patient level was considered successful. This threshold was chosen based on a review of previous literature comparing different strategies of heparin-free haemodialysis [2]. Data were analysed according to an intention-to-treat (ITT) analysis (number of successful sessions/scheduled sessions) and as treated (number of successful sessions/effectively performed sessions). Assuming a confidence level of 95%, an expected success proportion of 80% and a total width of the confidence interval of 15%, and assuming a normal approximation, a sample size of 109 sessions would be enough. We therefore aimed to include 120 sessions per arm for analysis. Second, both study arms were compared. As a secondary endpoint, time to clotting was analysed and plotted according to the Kaplan–Meier method. Differences between reduction ratios were analysed using paired t-test or Wilcoxon signed-rank test, depending on the distribution of variables. We considered P = 0.05 as a threshold of significance. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) and SPSS version 23 (IBM, Armonk, NY, USA).
RESULTS
Baseline demographics and patient flow
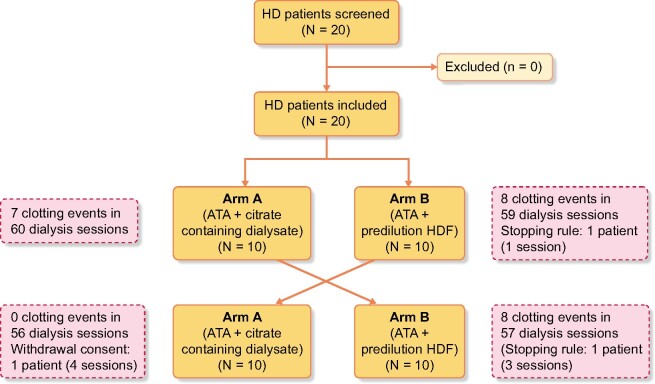
We performed an open-label, randomized, crossover trial to study the efficacy of two systemic heparin-free anticoagulation strategies in combination with an ATA dialyser. The study was registered with ClinicalTrials.gov (NCT04381234). A total of 20 patients provided informed consent. Patient inclusion and treatments were performed in the first quarter of 2019 (first patient first visit 14 January 2019, last patient last visit 9 March 2019). Baseline demographics are given in Table 1. All patients were scheduled for 6 sessions in each arm or 12 study sessions per patient. All patients took part in both treatment arms according to the protocol. Of 240 scheduled study dialysis sessions, 232 dialysis sessions were performed according to the protocol. One patient missed four sessions of Arm A (ATA dialyser plus citrate-containing dialysate) due to dropout (withdrawal of consent). For Arm B (ATA dialyser with high-volume predilution HDF), two patients missed four sessions in total, both after reaching the stopping rule (clotting in three consecutive sessions with the same study arm). Total dropout amounted to 3.33% (Figure 1).
Table 1.
Study population
| Variable | Values |
|---|---|
| Age (years), mean (SD) | 75.1 (12.3) |
| Gender (male/female), n/n | 9/11 |
| Dialysis vintage (months), median (minimum–maximum) | 32 (5–172) |
| Haemoglobin (g/dL), mean (SD) | 10.0 (1.1) |
| Thrombocytes (×109/L), mean (SD) | 210 (66) |
| Calcium (mmol/L), mean (SD) | 1.11 (0.07) |
| Magnesium (mmol/L), mean (SD) | 0.92 (0.11) |
| Anticoagulation, % | |
| Acetylsalicylic acid | 80 |
| Clopidogrel | 5 |
| Vitamin K antagonists | 0 |
| Non-vitamin K oral anticoagulants | 0 |
Relevant demographic, biochemical and drug therapy data. For drug therapy, the percentage of prescribed drugs at any time point (overall) and per study period is given.
Primary endpoint analysis
The primary endpoint of this study was completion of the prescribed study sessions with a fixed duration of 240 min without severe clotting. Both study procedures performed better than the prespecified cut-off for therapy failure, defined as treatment success in <80% of sessions and/or <80% of patients.
Study procedure A consisted of the use of an ATA membrane plus citrate-containing dialysate. Of 120 scheduled sessions, 116 were performed, as one patient withdrew consent, and 109 of these were considered successful. This equals successful completion in 90.8% of sessions when analysed as ITT and 94% of as-treated sessions.
Study procedure B consisted of the use of an ATA membrane with high-volume predilution HDF. Of 120 scheduled sessions, 116 were performed, as the stopping rule was reached before the start of 4 planned sessions. In this arm, 83.3% (ITT) and 86.6% (as treated) of sessions were completed.
There were no significant differences between study arms, neither in the ITT (Fischer’s exact P = 0.12), nor in the as-treated analysis (Fischer’s exact P = 0.07).
Secondary efficacy endpoints
We analysed time to clotting. Early clotting (<2 h) of the extracorporeal circuit was seldom, but occurred with both study arms. Early clotting was observed in one session in Arm A (combination ATA and citrate-containing dialysate) and in four sessions in Arm B (high-volume HDF with ATA membrane). When plotted as a Kaplan–Meier estimate, time to clotting was borderline significantly different between the study arms (Mantel–Cox log rank P = 0.05) in favour of Arm A (Figure 2).
FIGURE 1:
Study flow chart describing patient flow during study participation.
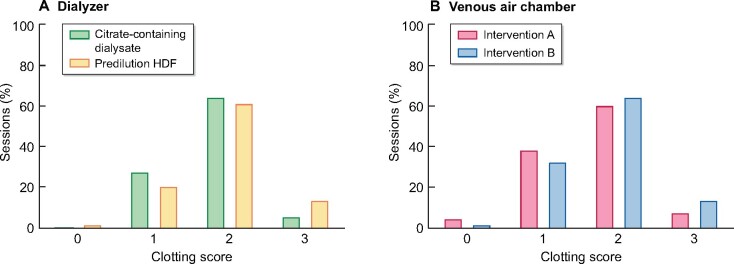
Previous studies have shown the validity of this semi-quantitative scoring system [10, 14]. We observed no significant differences in dialyser and venous air chamber clotting scores (Figure 3).
FIGURE 2:
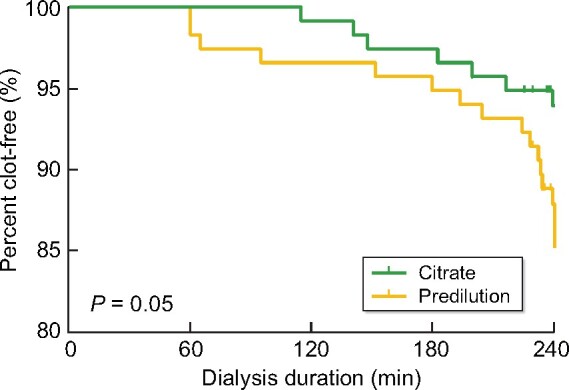
Kaplan–Meier curve of time to clotting. Time to clotting was not significantly different (P = 0.05).
Solute clearances were expressed as reduction ratios and for urea were also expressed as single-pool Kt/Vurea (Table 2). Dialysis with an ATA membrane and citrate-containing dialysate (Arm A) tended to be associated with higher clearances of small water-soluble solutes (P = 0.05). We observed significantly higher reduction ratios for the larger ‘middle’ molecules of β2-microglobulin (P = 0.0009) and myoglobin (P = 0.0001) in the patients treated with an ATA membrane and high-volume HDF. The reduction ratio of the protein-bound solutes indoxyl sulphate and p-cresol sulphate were similar in both arms.
Table 2.
Results
| Characteristics | ATA dialyser + | ATA dialyser + | P-value |
|---|---|---|---|
| citrate-containing dialysate | predilution HDF | ||
| Access type | |||
| AVF/AVG/CVC, n/n/n | 19/1/0 | 19/1/0 | NS |
| Sessions performed/scheduled, n/n | 116/120 | 116/120 | NS |
| Blood volume (L), mean (SD) | 74.44 (5.28) | 74.33 (4.32) | NS |
| Ultrafiltration volume (mL), mean (SD) | 1234 (764) | 1406 (811) | NS |
| Substitution volume (L), mean (SD) | 0 | 52.7 (11.1) | PP |
| Dialysate | |||
| Temperature (°C), median (25th–75th percentile) | 36 (35.5–36.5) | 36 (35.5–36.5) | NS |
| Na+ (mmol/L), range | 136–140 | 136–140 | NS |
| K+ (mmol/L), range | 1–3 | 1–3 | NS |
| (mmol/L) | 35 | 35 | NS |
| Ca2+ (mmol/L) | 1.5 | 1.25 | NS |
| Mg2+ (mmol/L) | 0.5 | 0.5 | NS |
| Citrate3− (mmol/L) | 1 | 0 | PP |
| Single-pool Kt/V, mean (SD) | 1.63 (0.26) | 1.51 (0.25) | 0.05 |
| Reduction ratios | |||
| Water-soluble | Blood urea nitroge | ||
| n (%), mean (SD) | 79.4 (5.7) | 77.1 (5.7) | 0.05 |
| Middle molecules (%), median (25th–75th percentile) | |||
| Β2-microglobulin | 66 (63–72) | 71 (67–76) | 0.0009 |
| Myoglobin | 61 (55–65) | 66 (62–72) | 0.0001 |
| Protein-bound solutes (%), mean (SD) | |||
| Indoxyl sulphate | 45.6 (10.7) | 46.3 (8.1) | NS |
| p-cresol sulphate | 40.3 (10.7) | 39.5 (8.7) | NS |
Characteristics of the dialysis prescription with both study arms.
AVF: arteriovenous fistula; AVG: arteriovenous graft; CVC: central venous catheter; NS: not significant; PP: per protocol.
Secondary safety endpoint
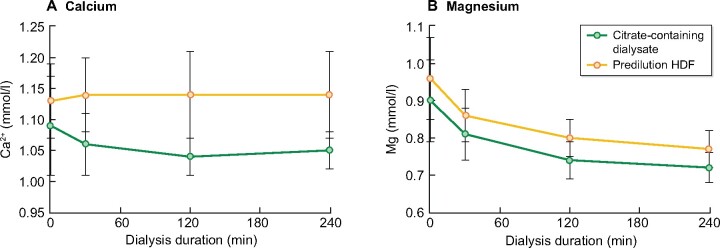
Hypocalcaemia is a well-known risk associated with the use of citrate. Previous studies have demonstrated that the risk of severe hypocalcaemia is negligible when citrate and calcium are both added to the dialysate. We measured serum ionized calcium in the arterial line of the dialysis tubing. In both arms, the mean ionized calcium concentrations were well within the acceptable range (Figure 4A). No patient developed clinically relevant hypocalcaemia.
FIGURE 3:
Clotting scores. Distribution of clotting scores of (A) dialyser and (B) venous air chamber. Although the distribution suggests that clotting scores are higher with Arm B, i.e. high-volume predilution HDF, than with Arm A, no significant differences were noted.
FIGURE 4:
Secondary safety endpoints. Evolution over time for (A) ionized calcium concentration (mmol/L) and (B) serum magnesium concentration (mmol/L).
We additionally monitored magnesium concentrations. As anticipated, we saw a decline over time in Arm A. However, we equally observed a decline in Arm B. No significant differences were noted (Figure 4B).
DISCUSSION
In this pilot Phase II crossover trial, we compared two different approaches of systemic heparin-free dialysis using the ATA membrane. We found that both the citrate-containing dialysate and high-volume predilution HDF resulted in acceptable rates of clot-free dialysis when performed with an ATA dialyser.
Heparin and LMWH are the standard-of-care anticoagulation regimen for extracorporeal blood circulation. There is a clear trade-off between maintaining the patency of the extracorporeal circuit and the associated risk of systemic anticoagulation contributing to an elevated bleeding risk. In these patients there is a clinical need for alternative anticoagulation regimens. In addition, a small subset of patients suffers from other side effects, e.g. heparin-induced thrombocytopaenia.
The last decade has seen a number of studies exploring different approaches to perform systemic heparin-free dialysis. These include saline infusion [8], citrate-containing dialysate [12, 13], regional citrate anticoagulation [11, 20] and heparin-coated membranes [8]. We recently studied the combination of a heparin-coated membrane and citrate-containing dialysate, with a success rate of 94% [1]. The combination of a heparin-coated membrane plus citrate-containing dialysate is clearly superior to the use of a heparin-coated membrane without adjunctive measures to prevent clotting [2]. Although this combination resulted in low rates of clotting, heparin-coated membranes are not ubiquitously available.
ATA dialysers have a low degree of platelet contact activation and might be an alternative to heparin-coated dialysers. In this study, we show that ATA dialysers have a low clotting propensity. We used a prespecified cut-off of at least 80% successful sessions, as we believe that any anticoagulation regimen that does not meet this threshold should be considered below par. Both study arms, i.e. the use of an ATA membrane plus citrate-containing dialysate and the use of an ATA membrane plus high-volume predilution HDF, can be considered adequate, as the success rate exceeds this threshold in both study arms. In a time-to-clotting analysis, the combination with citrate-containing dialysate appears to perform slightly better, although the results are only borderline significant.
The observed success rates suggest that the ATA dialyser might be an alternative to a heparin-grafted dialyser, especially in countries were heparin-grafted dialysers are not available. Indeed, the patient characteristics in this study are quite comparable to those of the CiTrate and EvoDial (CiTED) study, which compared the combination of a heparin-grafted membrane in combination with citrate-containing dialysate to regional citrate anticoagulation [1]. However, no head-to-head comparative data are available.
It is well-known that the dialytic clearance of different uraemic retention solutes is determined by the inverse of the hydrophilic radius as well as by the relative contribution of diffusion and convection [21–23]. As Arm B adds high-volume convective transport, we explored differences in the dialytic clearance of various uraemic retention solutes. As expected, we observed significantly higher reduction ratios for the larger molecules β2-microglobulin and myoglobin with high-volume HDF. Contrary to expectations, the urea reduction ratio (URR) and single-pool Kt/V were (borderline) significantly higher when treated with the ATA dialyser plus citrate-containing dialysate. We also did not observe significant differences between the study arms for the protein-bound uraemic retention solutes indoxyl sulphate and p-cresol sulphate.
The observed findings for small solute clearance and protein-bound solutes are not in line with previous empirical clearance studies [20]. In this study we measured reduction ratios and we do not have data on total solute removal. While this may explain why we do not observe significant differences for the protein-bound solutes, this does not provide an explanation for the significantly higher URR and single-pool Kt/V. Blood flow, known to have a profound impact on URR, was similar between arms (Table 2). One might speculate that with predilution HDF without anticoagulation there is more fouling of the membrane surface, but we did not observe this clinically. In the absence of a definite explanation, these data warrant confirmation in other studies.
This study has several limitations. This study included patients on stable maintenance dialysis and it is unclear whether these findings can be extrapolated to patients in need of acute haemodialysis. Second, as these were stable maintenance dialysis patients, the majority of patients (85%) was taking antiplatelet drugs during the study period. This may have affected the results of this study. Due to the crossover design, however, the effect was balanced between the study arms. Finally, this was a pilot study with the ATA dialyser used in both arms. While these data suggest that performance of this membrane is comparable to that of heparin-grafted dialysers, we need a head-to-head comparison study to definitely demonstrate equivalence.
In conclusion, both citrate-containing dialysate and high-volume predilution HDF in combination with ATA dialysers have a low clotting propensity. A head-to-head comparison study is required to demonstrate that ATA in combination with citrate-containing dialysate would be a suitable alternative to heparin-coated membranes for systemic heparin-free haemodialysis.
FUNDING
This was an investigator-driven study. The Department of Nephrology, UZ Leuven, Belgium, received a restricted grant from Nipro Europe to cover part of the expenses related to this study, including costs related to biochemical analyses as well as ATA dialysers. Part of this work has been presented at the American Society of Nephrology Renal Week, 5–10 November 2019, Washington, DC, USA.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Meijers B, Metalidis C, Vanhove T. et al. A noninferiority trial comparing a heparin-grafted membrane plus citrate-containing dialysate versus regional citrate anticoagulation: results of the CiTED study. Nephrol Dial Transplant 2017; 32: 707–714 [DOI] [PubMed] [Google Scholar]
- 2. Meijers BK, Poesen R, Evenepoel P.. Heparin-coated dialyzer membranes: is non-inferiority good enough? Kidney Int 2014; 86: 1084–1086 [DOI] [PubMed] [Google Scholar]
- 3. Davenport A. What are the anticoagulation options for intermittent hemodialysis? Nat Rev Nephrol 2011; 7: 499–508 [DOI] [PubMed] [Google Scholar]
- 4. Aggarwal A, Whitaker DA, Rimmer JM. et al. Attenuation of platelet reactivity by enoxaparin compared with unfractionated heparin in patients undergoing haemodialysis. Nephrol Dial Transplant 2004; 19: 1559–1563 [DOI] [PubMed] [Google Scholar]
- 5. Huang KW, Leu HB, Luo JC. et al. Different peptic ulcer bleeding risk in chronic kidney disease and end-stage renal disease patients receiving different dialysis. Dig Dis Sci 2014; 59: 807–813 [DOI] [PubMed] [Google Scholar]
- 6. Wu CY, Wu MS, Kuo KN. et al. Long-term peptic ulcer rebleeding risk estimation in patients undergoing haemodialysis: a 10-year nationwide cohort study. Gut 2011; 60: 1038–1042 [DOI] [PubMed] [Google Scholar]
- 7. Genovesi S, Rossi E, Gallieni M. et al. Warfarin use, mortality, bleeding and stroke in haemodialysis patients with atrial fibrillation. Nephrol Dial Transplant 2015; 30: 491–498 [DOI] [PubMed] [Google Scholar]
- 8. Laville M, Dorval M, Fort RJ. et al. Results of the HepZero study comparing heparin-grafted membrane and standard care show that heparin-grafted dialyzer is safe and easy to use for heparin-free dialysis. Kidney Int 2014; 86: 1260–1267 [DOI] [PubMed] [Google Scholar]
- 9. Guery B, Alberti C, Servais A. et al. Hemodialysis without systemic anticoagulation: a prospective randomized trial to evaluate 3 strategies in patients at risk of bleeding. PLoS One 2014; 9: e97187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Evenepoel P, Dejagere T, Verhamme P. et al. Heparin-coated polyacrylonitrile membrane versus regional citrate anticoagulation: a prospective randomized study of 2 anticoagulation strategies in patients at risk of bleeding. Am J Kidney Dis 2007; 49: 642–649 [DOI] [PubMed] [Google Scholar]
- 11. Richtrova P, Rulcova K, Mares J. et al. Evaluation of three different methods to prevent dialyzer clotting without causing systemic anticoagulation effect. Artif Organs 2011; 35: 83–88 [DOI] [PubMed] [Google Scholar]
- 12. Sands JJ, Kotanko P, Segal JH. et al. Effects of citrate acid concentrate (citrasate®) on heparin N requirements and hemodialysis adequacy: a multicenter, prospective noninferiority trial. Blood Purif 2012; 33: 199–204 [DOI] [PubMed] [Google Scholar]
- 13. Richtrova P, Mares J, Kielberger L. et al. Citrate-buffered dialysis solution (Citrasate) allows avoidance of anticoagulation during intermittent hemodiafiltration-at the cost of decreased performance and systemic biocompatibility. Artif Organs 2017; 41: 759–766 [DOI] [PubMed] [Google Scholar]
- 14. Evenepoel P, Maes B, Vanwalleghem J. et al. Regional citrate anticoagulation for hemodialysis using a conventional calcium-containing dialysate. Am J Kidney Dis 2002; 39: 315–323 [DOI] [PubMed] [Google Scholar]
- 15. Aoyagi S, Abe K, Yamagishi T. et al. Evaluation of blood adsorption onto dialysis membranes by time-of-flight secondary ion mass spectrometry and near-field infrared microscopy. Anal Bioanal Chem 2017; 409: 6387–6396 [DOI] [PubMed] [Google Scholar]
- 16. Vanommeslaeghe F, De SF, Josipovic I. et al. Evaluation of different dialyzers and the impact of predialysis albumin priming in intermittent hemodialysis with reduced anticoagulation. Kidney Int Rep 2019; 4: 1538–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swartz RD. Hemorrhage during high-risk hemodialysis using controlled heparinization. Nephron 1981; 28: 65–69 [DOI] [PubMed] [Google Scholar]
- 18. De Loor H, Poesen R, De Leger W. et al. A liquid chromatography–tandem mass spectrometry method to measure a selected panel of uremic retention solutes derived from endogenous and colonic microbial metabolism. Anal Chim Acta 2016; 936: 149–156 [DOI] [PubMed] [Google Scholar]
- 19. Gotch FA, Sargent JA.. A mechanistic analysis of the National Cooperative Dialysis Study (NCDS). Kidney Int 1985; 28: 526–534 [DOI] [PubMed] [Google Scholar]
- 20. Opatrny K, Richtrova P, Polanska K. et al. Citrate anticoagulation control by ionized calcium levels does not prevent hemostasis and complement activation during hemodialysis. Artif Organs 2007; 31: 200–207 [DOI] [PubMed] [Google Scholar]
- 21. Bammens B, Evenepoel P, Verbeke K. et al. Removal of the protein-bound solute p-cresol by convective transport: a randomized crossover study. Am J Kidney Dis 2004; 44: 278–285 [DOI] [PubMed] [Google Scholar]
- 22. Meyer TW, Leeper EC, Bartlett DW. et al. Increasing dialysate flow and dialyzer mass transfer area coefficient to increase the clearance of protein-bound solutes. J Am Soc Nephrol 2004; 15: 1927–1935 [DOI] [PubMed] [Google Scholar]
- 23. Meyer TW, Walther J, Pagtalunan ME. et al. The clearance of protein-bound solutes by hemofiltration and hemodiafiltration. Kidney Int 2005; 68: 867–877 [DOI] [PubMed] [Google Scholar]