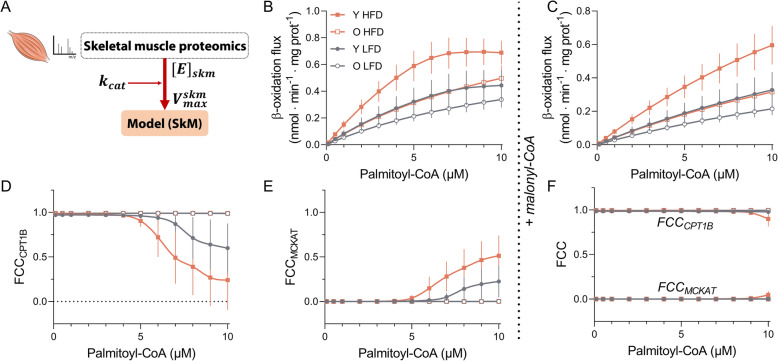
Fig. 5.
In silico β-oxidation simulations confirm flux dependence on CPT1B. a Schematic representation of the strategy used to parameterize the computational model of mitochondrial β-oxidation to the skeletal muscle. Proteomics data from the liver previously published were used to estimate kcats for the proteins involved in the pathway and applied to proteomics data obtained from quadriceps samples to calculate Vmaxs for each animal. b: Estimated steady-state β-oxidation fluxes (defined as the fluxes through CPT1B) based on in vivo-like conditions for different concentrations of substrate (palmitoyl-CoA) ranging from 0.1 to 10 μM and normalized for total homogenate protein. c Estimated steady-state β-oxidation-fluxes in the presence of 0.2 μM malonyl-CoA. Flux control coefficients (FCC) were calculated for the same substrate range. FCC is defined as ∂Jss/∂Ei, which represents to what extent an enzyme ‘i’ (Ei) can alter the steady-state flux of a pathway (Jss). FCCs for CPT1B (d) and MCKAT (e) are depicted. f FCCs for CPT1B and MCKAT in the presence of 0.2 μM malonyl-CoA. All simulations were performed for the Vmaxs estimated for each mouse in the dataset. Y stands for young (LFD: grey full circles; HFD: orange full squares) and O for old (LFD: grey open circles; HFD: orange open squares). Data are shown as mean ± SEM and were analysed using two-way ANOVA, n = 5–6 (young groups) or 10 (old groups). A complete description of the model and the assumptions made can be found in Additional file 6. Supporting data values can be found in Additional file 10.