Fig. 2.
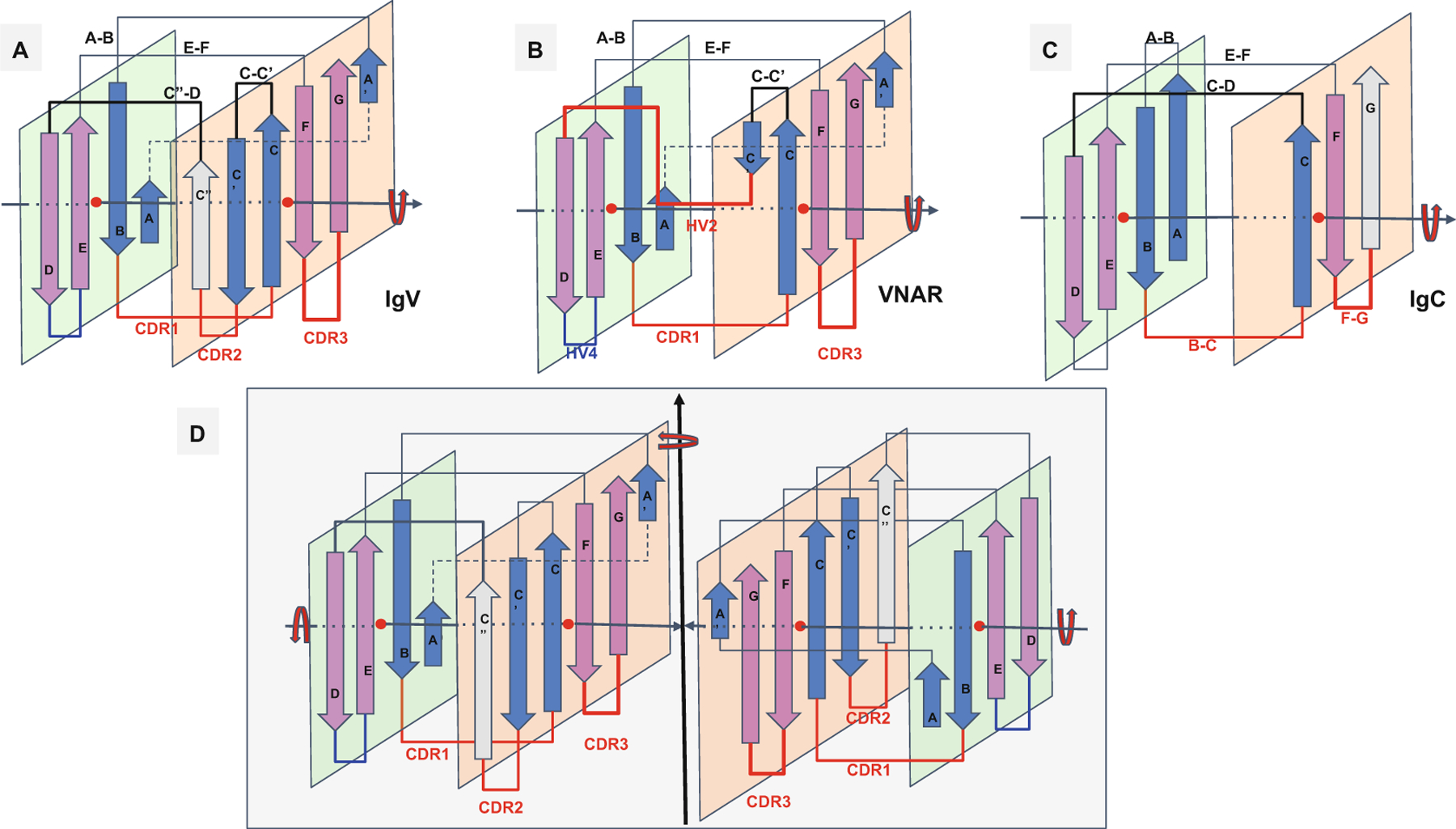
Ig domain topologies for IgV, Shark VNAR, and IgC. (a) In IgV domains, the A strand, with a flexible hinge in the middle, usually a cis-proline or a stretch of glycines, swaps the upper part of the strand from Sheet A to Sheet B in a parallel model. So-called domain swaps, which are most often SSE swaps among symmetric packing pairs of domains, are observed ubiquitously. Here we can refer to it as a protodomain (half-strand) swap by analogy. The linker between protodomains in this example of an IgV type domain forms a C′′ strand as an extension of Sheet B and the CDR2 loop between C′ and C′′, as well as a loop C′′-D bridging Sheet B back to Sheet A. (b) VNAR shows that same domain-level organization with two protodomains, yet a much smaller inter-protodomain linker, eliminating the linker’s supersecondary structure and the CDR2 loop. Instead, a short HV2 linker is observed. In the literature, C′ is usually included in the HV2 region, as it is very short. In addition, a hydrophilic set of residues on Sheet B, i.e., strands G|F||C|C′, facing out rather than hydrophobic in IgV, do not permit the formation of a symmetric dimer (as in D). This may also be due in part to the absence of an overall supersecondary structure of the linker in IgV (including C′), which may help patching an otherwise possibly semi-open eight-stranded barrel. (c) IgC. Here we consider only the IG C1-set, i.e., the antibody constant domain-like to exemplify an Ig constant domain protodomain connectivity. In this case the final domain is formed by a full four-stranded A|B||E|D Sheet A, with no half swapping of strand A, vs. a three-stranded G|F||C Sheet B, no C′ strand. Interestingly this enables C-domain-level dimerization through that four-stranded Sheet A as opposed the IgV dimer interface obtained through Sheet Bs, enabling a further helical level symmetric arrangements of chained Ig domains. When looking at an IgC protodomain alignment, only three strands are considered. (d) IgV dimer. In CD8aa, two IgV domains pack together symmetrically as homodimers through their Sheet B (G|F||C|C′) facing out form an eight-stranded semi-closed central barrel, with external strands C′ and G of two domains closing the central (quaternary) barrel symmetrically. In CD8ab, as in IgV light and heavy chain quaternary assembly, they pack pseudosymmetrically as heterodimers (see Figs. 3, 4, and S1). As the heterogeneity of domains increases, and even if a pseudosymmetry is maintained at the sheet level, packing, i.e., quaternary interface, becomes more asymmetric, and central barrels become open with an asymmetrical arrangement between “closing strands” C/G, resulting in at least one side of the central dimer barrel open. This is the case of a PD1-PDL1 pair (see Fig. 4)
