Figure 1.
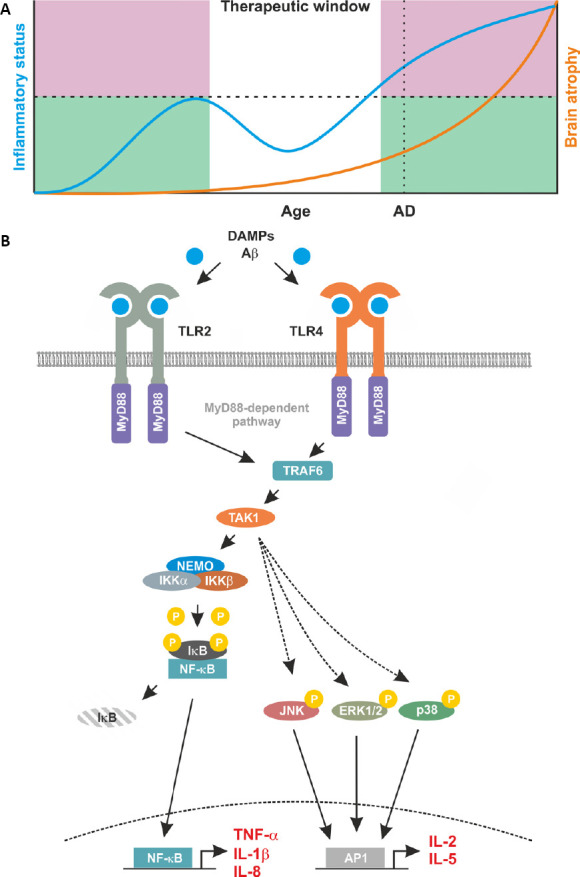
Proposed relationship between inflammation and brain atrophy and proinflammatory signaling induced by TLR2 and TLR4.
(A) A proposed temporal schematic of inflammatory status and disease progression in Alzheimer’s disease (AD). This profile indicates the progressive nature of a degeneration disease with age as indicated by brain atrophy (orange line, right axis), AD highlights a likely diagnosis of AD through clinical presentation. Alongside brain atrophy, inflammatory status is mapped (blue line, left axis). Here, an initial increase in inflammatory status is observed to counteract disease related damage. This increase is curtailed by the immune system and represents the ‘good’ inflammation (green shaded area). However, in patients, a secondary increase is observed. This escalates unchecked by the immune system and subsequently contributes to the degenerative processes. This ‘bad’ inflammation is indicated by the pink shaded area. This leaves a discrete therapeutic window, in which targeting the immune response may be beneficial for neurodegenerative diseases such as AD. (B) Proinflammatory feed forward loops mediated by TLR2 and TLR4. In AD, Aβ and DAMPs bind to and activate TLR2 and TLR4. Membrane-bound TLR2 and TLR4 recruit MyD88 and TRAF6 into to complex. This leads to the activation of TAK1 and in turn of the IKK complex composed of IKKα, IKKβ and NEMO. Once activated by upstream signals, IKKβ phosphorylates inhibitory IκB proteins thereby mediating their proteasomal degradation. Degradation of IκB liberates the nuclear localisation signals of DNA binding NF-κB subunits leading to their nuclear translocation and transcription of the pro-inflammatory target genes including TNF-α, IL-1β and IL-8. Once secreted, these proteins bind to their respective receptors further increasing NF-κB activity. This pro-inflammatory loop also involves a TAK1-mediated activation of the kinases JNK, ERK1/2, and p38. This results in activity of the transcription factor AP1 with additional pro-inflammatory targets including IL-2, and IL-5.
