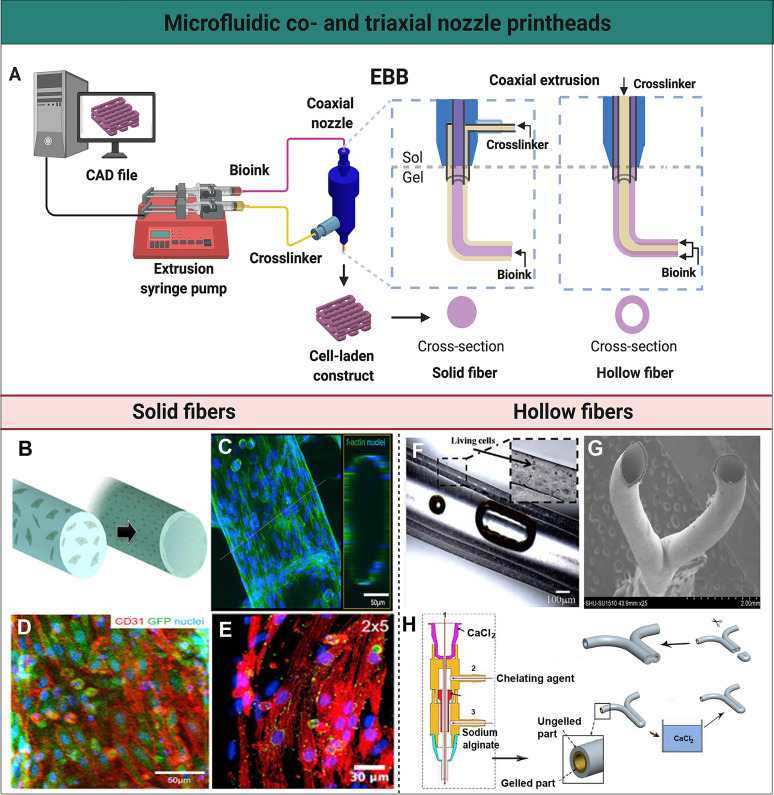
FIG. 2.
Microfluidic co- and triaxial printheads for the fabrication of solid and hollow fibers. (a) A schematic illustration of a microfluidics-enhanced EBB setup with a coaxial extruder. The mechanical-based extrusion syringe pump controls the dispensation of the bioink to create solid or hollow fibers, or their spatial patterns, in a controlled manner based on the CAD design. (b) A schematic representation of solid fibers encapsulating HUVECs that migrate to the periphery to form a lumen-like structure. (c) High-resolution confocal fluorescence micrograph visualizing the distribution of HUVECs on the endothelialized solid fiber. (d) Confocal fluorescence micrograph demonstrating green fluorescent protein (GFP)-HUVECs expressing the surface marker CD31 (red) indicating tight junction-formation. (e) A micrograph showing immunofluorescence staining of the post-seeded directionally aligned cardiomyocytes expressing sarcomeric α-actin (red) and connexin-43 (green). (b)–(e) Reprinted with permission from Zhang et al., Biomaterials 110, 45 (2016). Copyright 2016 Elsevier. (f) Perfusion of the medium through a hollow channel containing living cells. Reprinted with permission from Zhang et al., Nanotechnol. Eng. Med. 4, 0202902 (2013). Copyright 2013 American Society of Mechanical Engineers. (g) Scanning electron microscopy image of a Y-shaped hollow bifurcation. (h) Process of the fabrication of a Y-shaped hollow structure with a triaxial nozzle containing (1) a CaCl2 solution, (2) a chelating agent, and (3) sodium alginate. A Y-shaped hollow fiber was fabricated with this triaxial nozzle, subsequently crosslinked in a CaCl2 bath, and cut to form a branched structure. (g) and (h) Reprinted with permission from Li et al., Biomicrofluidics 10, 64104 (2016). Copyright 2016 IOP Publishing Ltd.