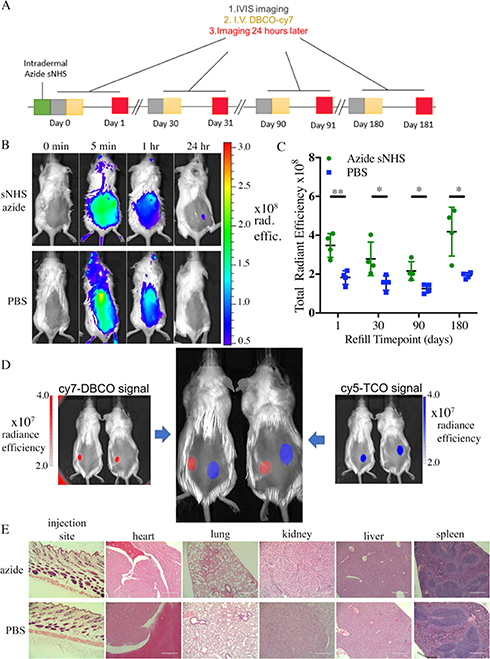
Figure 5.
Azide-sNHS ester depots allow long-term and repeated targeting with no apparent immunogenicity and are mutually compatible with tetrazine-TCO targeting for spatial separation of different regiments. (A) Timeline of systemic targeting of intradermal depots over long term. (B) Mice received an intradermal injection of azide-sNHS (50 μL of 0.2 M) or PBS and were administered iv DBCO-Cy7 and IVIS imaged before the dose and after 5 min, 1 h and 24 h. (C) Quantitation of systemic targeting of intradermal depots 1, 30, 90, and 180 days after intradermal injection of azide-sNHS (0.2 M, 50 μL) or PBS control. (D) 50 μL of methyltetrazine sNHS (right, 0.05 M) or azide-sNHS (left, 0.05 M) was injected intradermally on the dorsal flank of four mice. 1:1 DBCO-Cy7/TCO-Cy5 (200 μL) was injected iv (E) H&E staining of the skin injection site and major organs at 1 month for CD1 mice injected intradermally with 50 μL of azide-sNHS. Scale bar = 400 μm. Samples show mean ± standard error of the mean (SEM). *p < 0.05 by Student’s t-test. See Supporting Figures 7–10 for full IVIS images.