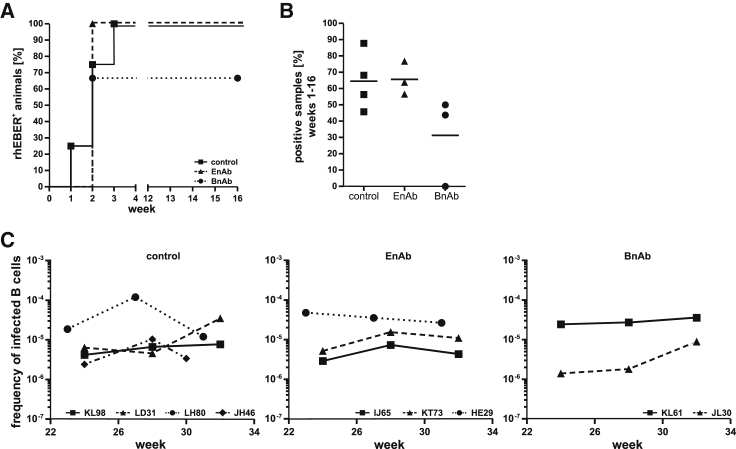
Figure 2.
Evaluation of acute and persistent infection after oral LCV challenge of animals infused with EnAbs, BnAbs, or a control mAb
(A and B) The percentage of animals in each group with at least one PBMC aliquot positive for rhLCV EBERs during the first 16 weeks after oral LCV challenge (A) and the overall percentage of PBMC aliquots positive for rhEBERs during the first 16 weeks (B); line indicates mean. The number of aliquots analyzed during the acute phase of infection (weeks 1–16) was 22–48 for all animals; see STAR Methods for details.
(C) The viral setpoint for individual animals as determined by limiting dilution analysis at three different time points during persistent infection (>16 weeks after viral challenge). Results are shown as the inverse number of peripheral blood B cells containing one LCV-infected B cell.