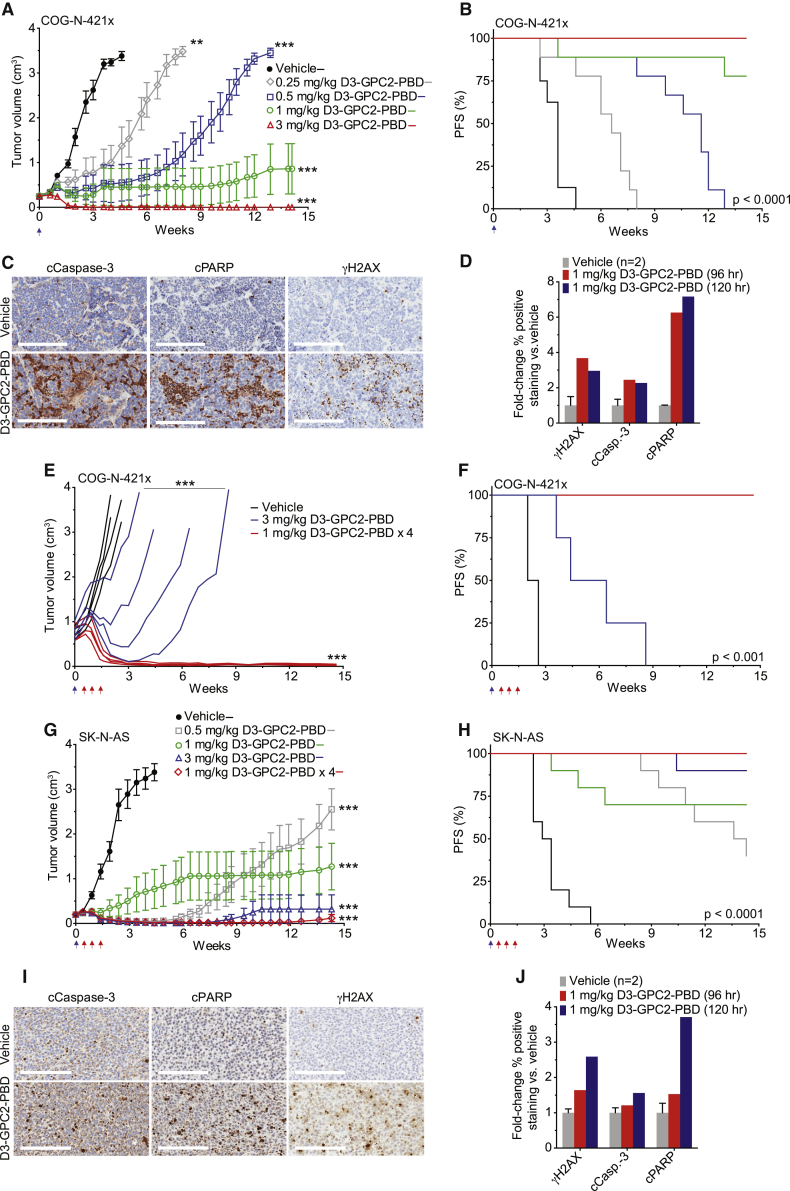
Figure 7.
D3-GPC2-PBD significantly extends the survival of mice with neuroblastoma COG-N-421x PDXs and SK-N-AS xenografts
(A) COG-N-421x PDX volumes after treatment with D3-GPC2-PBD (n = 8–9 mice/cohort; represented as mean ± SEM).
(B) PFS analysis of treatment arms in (A). Median survival of 3.6 weeks for vehicle, 6.6 weeks for 0.25 mg/kg ADC, 11.6 weeks for 0.5 mg/kg ADC, and >14.1 weeks for 1 and 3 mg/kg ADC treatment cohorts.
(C) IHC of COG-N-421x PDXs 5 days after treatment with 1 mg/kg D3-GPC2-PBD.
(D) Quantification of relative γH2AX, cleaved caspase-3, or cleaved PARP IHC staining after D3-GPC2-PBD treatment.
(E) Locally advanced COG-N-421x PDX volumes after treatment with D3-GPC2-PBD (n = 4 mice/cohort; starting mean tumor volumes = 0.75–0.77 cm3).
(F) PFS of treatment arms in (E). Median survival of 2.3 weeks for vehicle, 5.4 weeks for 3 mg/kg ADC, and >14.6 weeks for 1 mg/kg ADC × 4 treatment cohorts.
(G) Neuroblastoma SK-N-AS xenograft volumes after treatment with D3-GPC2-PBD (n = 10 mice/cohort; represented as mean ± SEM).
(H) PFS of treatment arms in (G). Median survival of 3.2 weeks for vehicle; 14.0 weeks for 0.5 mg/kg ADC; and >14.3 weeks for 1 mg/kg ADC, 1 mg/kg ADC × 4, and 3 mg/kg ADC treatment cohorts.
(I) IHC of SK-N-AS xenografts 5 days after treatment with 1 mg/kg D3-GPC2-PBD.
(J) Quantification of relative γH2AX, cleaved caspase-3, or cleaved PARP IHC staining after D3-GPC2-PBD treatment.
Scale bars: 200 μm in (C) and (I). Blue arrows in (A), (B), and (E)–(H) represent initial ADC dose, and red arrows in (E)–(H) indicate subsequent 3 ADC doses for the 1 mg/kg ADC × 4 treatment cohort.
∗∗p < 0.001; ∗∗∗p < 0.0001.
See also Figure S7.