Fig. 4 |. Intercellular glial signalling mechanisms that contribute to neuroplasticity during gastrointestinal inflammation.
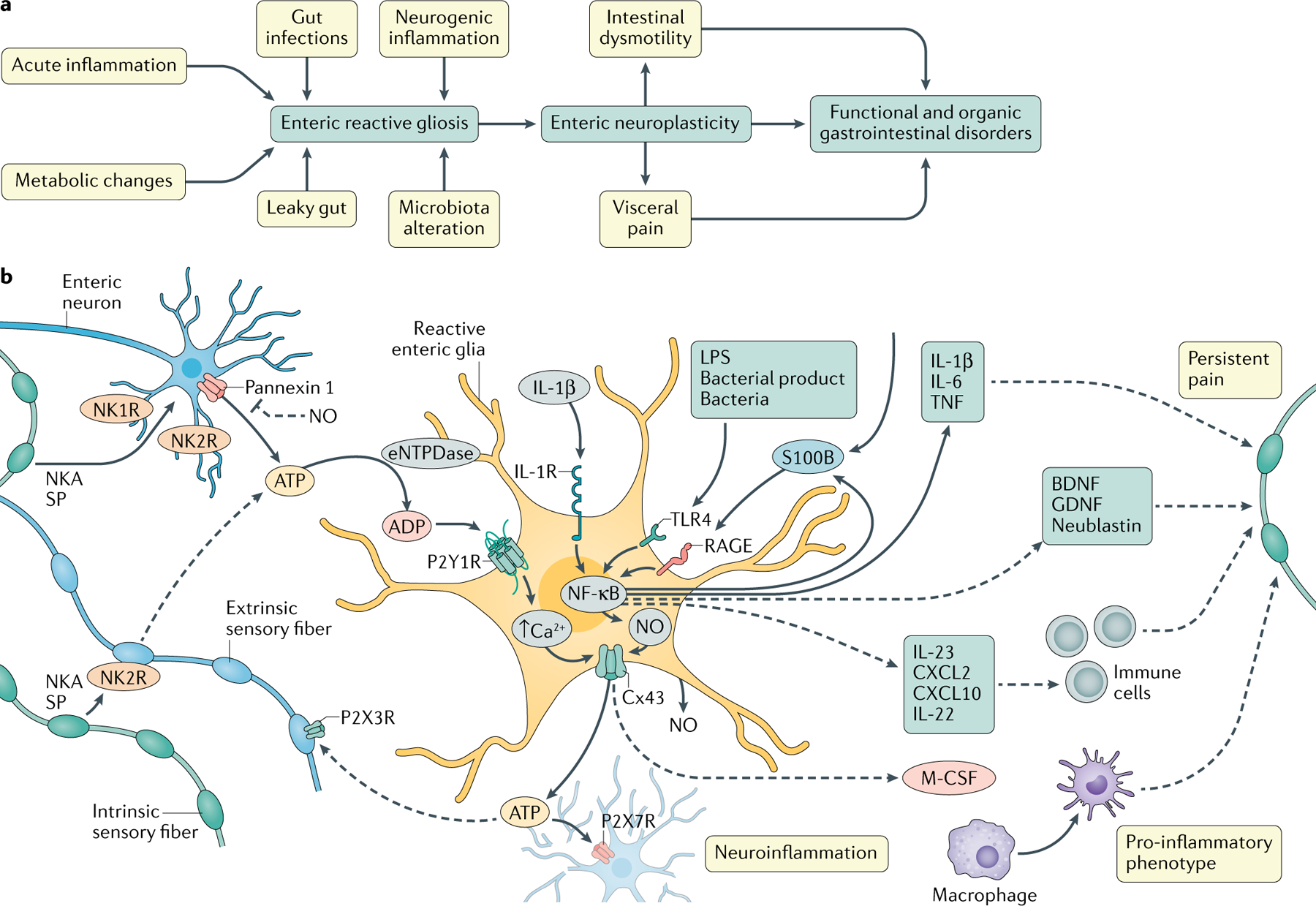
a | Reactive enteric gliosis is a protective response to potentially harmful stimuli. Reactive enteric glia contribute to functional abnormalities that underlie both functional and organic gastrointestinal disorders by promoting neuronal plasticity. b | Enteric glial signalling mechanisms active during acute inflammatory responses. Enteric reactive gliosis may be driven by adenosine triphosphate (ATP) release via pannexin 1 channels from enteric neurons intensively stimulated by neuronal mediators, such as neurokinin A (NKA), substance P (SP) or ATP. A self-perpetuating process derives from the activation of P2Y1Rs in the surrounding glial cells and their release of ATP through connexin 43 (Cx43) hemichannels. ATP released by glia drives P2X7R-mediated neuroinflammation and nociceptive neuron activation, likely via P2X3Rs. Glial activation also mediates the Cx43-dependent release of macrophage colony-stimulating factor (M-CSF) and other mediators that potentially regulate the activation of muscularis macrophages and visceral sensitivity in intestinal inflammation. Pro-inflammatory signals, such as S100β, IL-1β and bacterial products, increase the release of nitric oxide (NO) and other inflammatory signals via NF-κB that directly or indirectly affect the normal glia–neuron purinergic signalling and/or neuronal sensitivity through actions on local immune responses. eNTPDase, ectonucleoside triphosphate diphosphohydrolase 2; GDNF, glial cell-derived neurotrophic factor; LPS, lipopolysaccharide.
