Abstract
Purpose
To identify the contribution of mutations in the Desert Hedgehog (DHH) gene to the disorders of sexual differentiation (DSD) and male infertility.
Methods
The study included a total 430 subjects, including 47 gonadal dysgenesis cases, 6 patients with undescended testis and infertility characterized by azoospermia, 125 infertile male patients characterized by oligoasthenozoospermia, 24 patients with oligoasthenoteratozoospermia, and 200 ethnically matched normozoospermic fertile men who had fathered a child in the last two years. Sequencing of the complete coding region of the DHH gene was undertaken to find its contribution to the DSD and male infertility.
Results
We observed four novel mutations in the DHH gene in the cases with different reproductive anomalies. A synonymous substitution, c. 543C>T (p.His181His) was observed in 6.6% oligoasthenozoospermic infertile males and 1.5% normozoospermic fertile control samples (RR = 4.4077, 95%CI 1.19–16.29). Another synonymous substitution, c.990G>A (p.Ala330Ala) was observed in an infertile patient with unilateral undescended testis (case #12). Insertion of G at c.1156insG (p.Arg385fs) was observed in a case with bilateral undescended testis and azoospermia (case #23). In gonadal dysgenesis category, two mutations, insertion of G at c.1156insG (p.Arg385fs) and c.997A>G (p.Thr333Ala) substitution were observed in one case (case #34). These mutations were completely absent in control samples.
Conclusion
Mutations in the DHH gene impact reproduction with mild mutations affecting fertility, and severe or multiple mutations resulting in gonadal dysgenesis.
Keywords: DHH gene, Gonadal dysgenesis, Male infertility, Cryptorchidism
Introduction
Testis differentiation begins with SRY expression in the Sertoli cell precursors. The Sertoli cells eventually initiate the process of spermatogenesis. Although the complete cell-cell and molecular interactions in spermatogenesis are not yet elucidated, a few major molecular events have been deciphered. It has been shown in cell cultures that Sertoli-derived cell line supported meiotic progression of the germ cells [1, 2]. There are several signaling factors secreted during this process of germ cell development [1]. One of these, the desert hedgehog (Dhh) gene in mice starts expressing at the testis differentiation stage and continues to express in the adult testis [3, 4]. Out of three hedgehog family members, only DHH is expressed in the testis [5]. DHH signaling protein is produced by the Sertoli cells [6] and its receptor is localized on the cells of interstitium in 12.5dpc gonads, which suggests its role in the initiation of Leydig cell differentiation [7]. Its expression in spermatocytes and round spermatids in the adult mouse testis suggests its role in spermatogenesis [8, 9]. Hedgehog signaling transmits its signals via the activation of Gli transcription factors. In the absence of DHH ligand, inhibition of Smoothened (SMO); the full-length glioma associated oncogene (GliFL) undergoes proteolytic cleavage to Gli repressor (GliR) after phosphorylation. GliR represses the transcription of hedgehog target genes. On binding of hedgehog ligand to its receptor, the complex internalizes and is degraded by lysosomes, relieving SMO protein. The SMO protein through suppressor of fused (SUFU) protein signals the release of Gli activator, resulting in the activation of its target genes [10].
DHH gene in humans is located on 12q12-q13.1 and is composed of 3 exons encoding a protein of 396 amino acids [11]. Dhh expression (begins at 11.5 dpc) follows Sry expression (begins at 10.5 dpc). There is no expression of Dhh gene in female mice during this developmental stage or later [4]. This indicates the role of Dhh gene in the initial architectural organization of the testis. Dhh expression continues even in the later embryonic stages [4], where Sry expression is lost [12]. DHH signaling seems to be important for communication between the Sertoli and germ cells [4], which is critical to testicular development and spermatogenesis. Dhh continues to express even in the adulthood, indicating its plausible role in the initiation and maintenance of spermatogenesis [6]. The components of hedgehog signaling such as PTCH1, PTCH2, SMO, SUFU are present in both mitotic and meiotic germ cells. The increased expression of Sufu in the later stages of spermatogenesis, such as round spermatids and elongating spermatids adds to an active regulatory role of DHH signaling in spermatogenesis [5, 8]. Dhh knockout had no effect on female development and fertility, but male mouse lacked germ cells, had reduced Leydig cell number and were infertile [4, 7]. Similarly, ectopic expression of Gli1 resulted in meiotic arrest [13]. All the above-provided evidence suggests a critical role of DHH signaling in the early embryonic testicular development, spermatogenesis in the adult and male fertility.
Gonadal dysgenesis most often results from mutations in the genes participating in the early stages of testis differentiation. Apart from the SRY and SOX9 genes, mutations in the DHH have been identified in the gonadal dysgenesis patients [14–25]. However, its expression in the adult testis also makes it an ideal candidate for screening of mutations in male infertility. As mentioned above, DHH mutations have been explored in gonadal dysgenesis, but no study has been conducted on infertile male subjects or cases with reproductive anomaly other than gonadal dysgenesis. We hypothesized that mutations in the DHH gene could result in gonadal dysgenesis, loss of spermatogenesis or complete abolition of spermatogenesis without affecting the development of external genitals. Therefore, we undertook sequencing in the complete coding region of the DHH gene in the cases with partial/complete gonadal dysgenesis and infertile males with apparently normal external genitals and well-descended testis, and infertile males with unilateral or bilateral undescended testis.
Materials and methods
Subjects and clinical history
The patients were recruited through the Institute of Reproductive Medicine (IRM), Kolkata, India and King George’s Medical University, Lucknow, India. The patients reported to the hospital either due to sexual ambiguities, primary amenorrhea or male/female infertility. A detailed history of each patient was taken along with physical examination. In addition to the patient, information regarding the siblings was collected, wherever possible.
The study included a total 430 subjects, including 47 gonadal dysgenesis (19 patients characterized by complete gonadal dysgenesis, 28 patients with partial gonadal dysgenesis), 6 patients with undescended testis and infertility characterized by azoospermia, 125 infertile patients characterized by oligoasthenozoospermia, but with apparently normal external genitals and well-descended testis, 24 patients with oligoasthenoteratozoospermia and normal external genitals with well-descended testis, and 200 ethnically matched normozoospermic fertile men who had fathered a child in the last two years. Partial gonadal dysgenesis patients presented with female external genitals, blind ending vagina, rudimentary uterus, and abdominal gonads with testis on one side and undifferentiated gonad on the other. Individuals with complete gonadal dysgenesis presented with female external genitals, blind ending vagina, rudimentary uterus, and abdominal undifferentiated gonads on both sides. Four of the undescended testis cases had unilateral anomaly and two had bilateral anomaly. Peripheral blood samples were collected with informed written consent of each subject.
The classification of the infertile patients into groups was done according to the WHO 2010 criteria [26]. Infertility duration in these patients ranged from 2.5 to 7 years. Oligoasthenozoospermic cases were characterized by sperm count less than 15 million per ml and total motility less than 40%. Oligoasthenoteratozoospermia was defined as sperm count less than 15 million per ml, total motility less than 40%, and abnormal sperm shape <4%. All structural deformities, including the head, mid-piece, and tail were considered for defining teratozoospermia. Normozoospermic fertile men had sperm count >15 million per ml, total motility >40%, and normal forms >4%, considered normal as per the WHO 2010 criteria. In all infertile patients, female factors were ruled out upon a detailed standard clinical investigation including regularity of menstruation, ultrasonography for structural normalcy of female reproductive system, intravaginal sonography for follicular development for at least one menstrual cycle, hormone levels, BMI, and/or the presence of significant co-morbidities. Semen profiles of infertile cases and controls are provided in Table 1.
Table 1.
Semen parameters of infertile and control groups. Data are provided as mean + SD
| Parameters | OA infertile cases (N = 125) | OAT infertile cases (N = 24) | Controls (N = 200) | One-way ANOVA | Tukey’s post hoc test |
|---|---|---|---|---|---|
| Age (years) | 36 + 3.5 | 36 + 2.3 | 37 + 4.3 | P = 0.06 | OA vs OAT: P = 0.99 |
| OA vs Control: P = 0.07 | |||||
| OAT vs Control: P = 0.46 | |||||
| Semen volume (ml) | 2.8 + 1.48 | 3.1 + 1.54 | 2.9 + 1.39 | P = 0.61 | OA vs OAT: P = 0.62 |
| OA vs Control: P = 0.81 | |||||
| OAT vs Control: P = 0.79 | |||||
| Sperm count (million/ml) | 9 + 4.5 | 10 + 4.2 | 65 + 40 | P = 0.00 | OA vs OAT: P = 0.98 |
| OA vs Control: P = 0.00 | |||||
| OAT vs Control: P = 0.00 | |||||
| Sperm motility (%) | 23 + 9.5 | 16 + 12 | 63 + 16.5 | P = 0.00 | OA vs OAT: P = 0.07 |
| OA vs Control: P = 0.00 | |||||
| OAT vs Control: P = 0.00 | |||||
| Progressive motility (%) | 11 + 10.5 | 8 + 9.1 | 45 + 11 | P = 0.00 | OA vs OAT: P = 0.42 |
| OA vs Control: P= 0.00 | |||||
| OAT vs Control: P = 0.00 | |||||
| Normal morphology | >4% | 2% | >4% | – | – |
*OA, oligoasthenozoospermic; OAT, oligoasthenoteratozoospermic
Cytogenetic analyses
In order to rule out chromosomal abnormalities as the cause, cytogenetic analysis was undertaken in the cases with gonadal dysgenesis as per the protocol detailed in a previous study [27]. Briefly, peripheral blood lymphocyte cultures were set up in duplicate for all family members in 5-ml culture vials with Roswell Park Memorial Institute (RPMI) media (Sigma-Aldrich, Munich) supplemented with 10% fetal calf serum (Sigma-Aldrich, Munich). Cells were cultured in the presence of penicillin-streptomycin-gentamycin. Phytohemagglutinin was added to stimulate cell division. Dividing cells were arrested at the metaphase stage with colchicin and fixed in methanol and acetic acid (3:1). Fixed cells were dropped onto glass slides and allowed to air dry. Chromosomes were G-banded by treating the preparations with trypsin followed by staining with Giemsa (Sigma-Aldrich, Munich).
Gonadal histology in gonadal dysgenesis patients
To figure out gonadal dysgenesis, histology was performed in this group of patients as per the protocol detailed elsewhere [28]. Gonads were removed surgically in 19 patients characterized by complete gonadal dysgenesis and 28 patients with partial gonadal dysgenesis. Gonadal tissue was fixed with 10% buffered neutral formalin solution at room temperature for 7 days. Tissue was dehydrated with isopropanol, cleared with xylene at room temperature, and impregnated with paraffin wax at 58°C. Tissue embedded in paraffin wax was cut into 4-mm thick sections with a Leica RM2135 microtome (Leica, Germany). The sections were directly taken on egg albumin–coated glass slides and kept at 60°C for 1 hour. Slides were dewaxed with xylene and stained with hematoxylin followed by eosin. After staining, the slides were mounted with dipthyline xylene and observed with an Axioplan imaging system (Zieman, Zeiss, Germany). Images were captured at different magnifications.
Genetic analyses and DNA sequencing
DNA was extracted from peripheral blood by the protocol described in our earlier study [12]. The gonadal dysgenesis cases were ruled out for mutations in the SRY, SOX9, DAX1 and SF1 genes by sequencing the complete coding regions including exon-intron boundaries of these genes. Briefly, all exonic regions of these genes along with the exon-intron boundaries were amplified by PCR and subjected to Sanger sequencing for the identification of mutations. Similarly, infertile male individuals were ruled out for the Y-chromosome deletions, specifically for the markers recommended by the European Academy of Andrology (EAA) [29].
The complete coding region of the DHH gene along with exon-intron boundaries was amplified using primers detailed in Table 2. The amplicons were directly sequenced using dideoxy chain terminator cycle sequencing protocol (BigDyeTM) and ABI 3730 DNA Analyzer (Applied Biosystems, USA) [30]. Multiple sequence alignment, editing and consensus sequences building were undertaken using the AutoAssembler software (Applied Biosystems, USA). The sites of mutation were sequenced in three independent PCR products in forward and reverse directions to confirm the presence of each mutation.
Table 2.
The list of primers used for amplification of the coding region of the DHH gene
| Exon # | Sequence (5’-3’) |
|---|---|
| EXON 1 | F-ACACACTTCAGTCGCAAAATGGA |
| R- TCTTGTCCTCACTAACTCTCCTGT | |
| EXON 1 | F- TATGATGGTTTGTCAGTAGCAGGT |
| R- AGGCTAAACTCTCTGTAGGTGA | |
| EXON 2 | F- TAACAAAGAATCAACCCTCCT |
| R- TAGGGTGGCAACAGTACTACT | |
| EXON 3 | F- ATGACTGGCGATATCTGCGCT |
| R- AGCAAGAGGCCAGGACATCGTT | |
| EXON 3 | F- TTTGTGGCTGTGGAGACCGAGT |
| R- AGCCCCATATCTCAGCCAGCA | |
| EXON 3 | F- ATGCATTGGTACTCTCGGCT |
| R- TCCCAAAGACCCTGCTGAGGA |
Results
No cytogenetic abnormality in any case
Cytogenetic analyses of all gonadal dysgenesis patients revealed no numerical or structural chromosomal abnormality. All individuals with gonadal dysgenesis had a normal 46, XY karyotype in 80 karyotypes analyzed for each patient.
Histology
Out of a total 47 cases of gonadal dysgenesis, histology confirmed complete gonadal dysgenesis in 19 individuals and partial gonadal dysgenesis in 28 individuals. Partial gonadal dysgenesis patients presented abdominal gonads with testis on the one side and undifferentiated gonad on the other while individuals with complete gonadal dysgenesis presented abdominal undifferentiated gonads on both the sides.
DHH mutations in gonadal dysgenesis and infertility cases
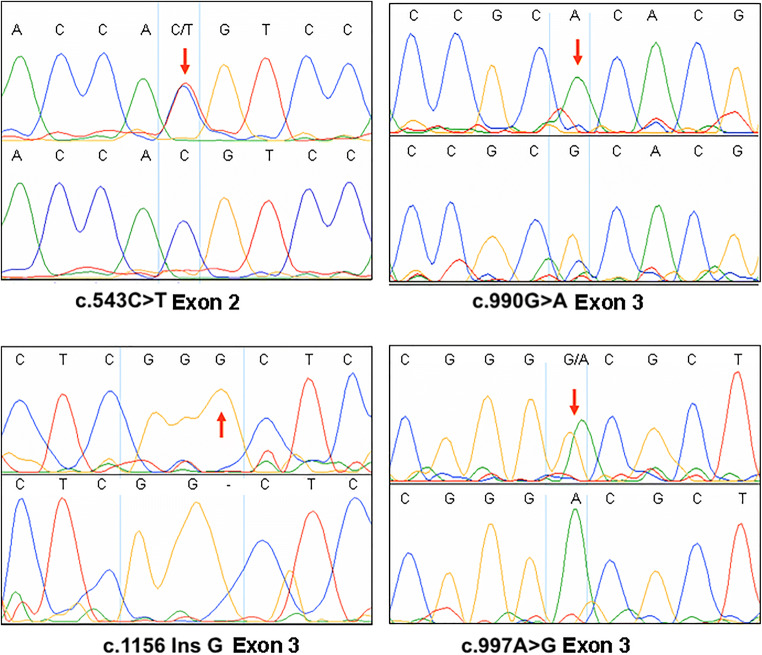
We sequenced the complete coding region of the DHH gene along with exon-intron boundaries. Four novel mutations in the DHH gene in the cases with different reproductive anomalies were observed (Fig. 1). A synonymous substitution, c. 543C>T (p.His181His) CAC-CAT, was observed in 6.6% (8 out of 121) oligoasthenozoospermic infertile males. This substitution was observed in 1.5% (3 out of 200) normozoospermic fertile control samples as well. The substitution conferred a risk ratio of 4.4077 (95% CI, 1.19–16.29). Another synonymous substitution, c.990G>A (p.Ala330Ala) GCG-GCA, was observed in an infertile patient with unilateral undescended testis (case #12). The patient had a normal male phenotype with apparently normal external genitals. The mutation was absent in control samples. Insertion of G at c.1156insG (p. Arg385fs) position in exon 3 was observed in a case (#23) with bilateral undescended testis and azoospermia. The patient had small penis and scrotum but was apparently normal otherwise. The insertion resulted in a frameshift beyond codon 385, and introduced delayed termination. This mutation was absent in the control samples. Gonadal dysgenesis is a higher degree of abnormality when compared with male infertility. In gonadal dysgenesis category, mutations were observed only in one case with external female genitals and poorly developed breasts, and complete gonadal dysgenesis (#34). We observed two mutations in this case: insertion of G at c.1156insG (p. Arg385fs) position in exon 3 (as reported above in an infertile man) and ACG-GCG c.997A>G (p.Thr333Ala) substitution. This mutation was completely absent in control samples.
Fig. 1.
Sanger sequencing electropherograms with mutation sites highlighted
Discussion
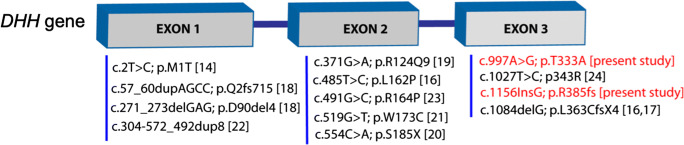
Umehara et al. (2000) identified the first mutation (missense ATG-ACG) in the initiation codon of the DHH gene in a patient having partial gonadal dysgenesis accompanied by minifascicular neuropathy. The patient described by Umehara et al. had minifascicular neuropathy similar to the peripheral nerve abnormalities seen in Dhh null mice [14]. Looking at the concordance of the phenotype between Dhh null mutant mice and human subjects, the authors concluded about the pathogenic nature of this mutation. The authors emphasized on the quantitative effects of Dhh mutations and added that a heterozygous mutation in this gene was insufficient to cause partial gonadal dysgenesis similar to the phenotype in heterozygous null mice. Later, Canto et al. (2004) reported two mutations in the complete gonadal dysgenesis patients, an L162P homozygous substitution in exon 2 in one patient and a homozygous 1084delG in exon 3 in two patients [16]. The same group in a later report identified the preceding mutation 1086delG in heterozygous form in two other patients with mixed gonadal dysgenesis [17]. This report further indicated that DHH mutations act in a dose-dependent manner. Homozygous and heterozygous mutations in the DHH gene in various degrees of gonadal dysgenesis, such as complete gonadal dysgenesis (CGD), partial gonadal dysgenesis (PGD), PGD with neuropathy, PGD with primary amenorrhea have been reported [14–25] (Fig. 2).
Fig. 2.
DHH mutations reported in gonadal dysgenesis cases
We observed a synonymous mutation (His181His) associated with infertility characterized by reduced sperm count (oligoasthenozoospermia). Another similar synonymous change GCG 330 GCA (Ala330Ala) was observed in an infertile man with partially descended testis in an individual who was otherwise healthy. Most of the synonymous mutations are either silent or cause little aberrance such that they remain silent. However, it has been reported that synonymous changes in codons may affect the stability of mRNA, thus affecting the level of the corresponding protein [31]. Not only this, synonymous changes in the codons have also been shown to differ in the translation efficiency due to codon usage preference [32]. For example, CAC codon for histidine is more efficiently used than CAT codon in E. coli. It is possible that such preferences exist in humans too. Another reason behind the molecular effects could be related to the generation of small or long non-coding RNAs from this locus. To analyze this possibility, we aligned all hairpin and mature miRNAs available at miRBase to the DHH gene, finding no miRNA originating from this locus. Analysis of lncRNAs using CNIT server (coding non-coding identifying tool) and Noncode Database (http://www.noncode.org) identified three lncRNAs (NONHSAT028069.2, NONHSAT028070.2, NONHSAT028071.2) originating from the DHH gene, of which at least one (NONHSAT028070.2) overlapped with c.543 C>T mutation. However, the impact of this change on lncRNA function remains unknown. We hypothesize that the partial loss of DHH function due to His181His and Ala330Ala substitutions could be due to codon usage preference, altered mRNA stability, or change in the function of regulatory RNAs originating from this gene, resulting in male infertility.
An insertion of G at 4944 position in the third exon resulted in a frameshift beyond codon 385, resulting in delayed termination. The frameshift resulted in the change of all amino acids beyond the point of insertion, causing a greater anomaly. Accordingly, this case (#13) presented with severe abnormalities characterized by undescended testis, smaller penis, and infertility (azoospermia). This mutation, in combination with another mutation, ACG 333 GCG (Thr333Ala), was observed in a case with complete gonadal dysgenesis (#14). The individual bearing these two mutations presented with female external genitalia, blind ending vagina, rudimentary uterus, and complete gonadal dysgenesis. The severe phenotypic effect in this individual was probably the result of more than one mutation introducing changes in the protein sequence. The severity of the effect in this case could also be due to the replacement of a polar amino acid (Thr) with a non-polar one (Ala). The association of synonymous mutations in the DHH gene with lesser degree of defect (male infertility), non-synonymous with higher degree of defect (male infertility with undescended testis), and non-synonymous mutation with even higher degree of anomaly (sex reversal with gonadal dysgenesis), suggests that DHH function mutations may result in quantitative loss of function. The varied effect of two synonymous substitutions in the present study may be due to different locations in the protein sequence, which defines the level of the loss of function due to a particular mutation. The preceding findings not only suggest the dosage dependent effect of DHH mutations, but also the role of this gene in proper testis differentiation, development of the external genitals, and the onset and maintenance of spermatogenesis.
Dhh null mutant mice have peripheral nerve abnormalities. These include perineurial cells ectopically located within the endoneurial space, which formed minifascicles around small groups of nerve fibers. Umehara et al., (2000) observed similar minifascicle formation in a partial gonadal dysgenesis patient with methionine-threonine (ATG-ACG) mutation. Later, Canto et al. (2004) reported two other mutations in this gene in gonadal dysgenesis; however, they reported no symptom of nerve dysfunction [16]. None of the patients in the present study had any symptom of nerve dysfunction. This may indicate that the location of mutation in this gene is probably more important in determining the loss of nerve function. It would also be interesting to explore if it is the extent of loss of DHH function that determines the nerve abnormality. However, no functional assay has been conducted till date to understand the relation between the degree of the loss of DHH function and gonadal dysgenesis or nerve phenotype.
We observed none of the previously reported DHH gene mutations in our cases. This may be due to ethnic variations in the samples analyzed. Since DHH signaling plays roles from the beginning of testis differentiation to adult testis during spermatogenesis, there are more opportunities for analyses of this gene in a variety of the cases presenting anomalies of the male reproductive system including infertility, ambiguous genitalia, and sex reversal with gonadal dysgenesis, etc. Crystal structure of the DHH protein is not available till date. We believe that the determination of crystal structure of DHH protein should help in explaining the phenotypes arising as a result of mutations in this gene. Our study shows that the prevalence of DHH mutations in the patients with disorders of sexual differentiation is low. This could be due to their direct impact on reproductive fitness and fertility. In the genes that affect fertility, the mutations are not inherited, and new mutations arise de novo. Only mild polymorphisms, which reduce sperm count may be inherited in fertile individuals or get transferred by assisted reproduction in infertile individuals. This is the first report of DHH mutations in male infertility and undescended testis cases, and none of the four mutations has been reported earlier. This opens the field for analysis of the DHH gene sequence variations in individuals with various male reproductive disorders in different populations.
It may be worth noting that in addition to the mutation, genetic background also affects the overall phenotype. Bitgood et al. (1996) reported variations in the Dhh null mice phenotype depending upon the genetic background of the animals [4]. The inbred animals of 129/Sv strain developed germ cells up to the primary spermatocyte stage, but germ cells in 129/Sv-C57BL/6JF1 hybrids developed up to step 15 spermatids. Similarly, Clark et al (2000) reported that Dhh null mice bred on a mixed background exhibited a phenotype with more severe abnormality than reported earlier [6]. It remains yet to be explored if ethnic variations in human subjects with DHH mutation(s) would affect overall phenotype in a manner similar to mice; nevertheless, the plausibility of this is quite high. Studies on patients with different ethnicities will be important for understanding this aspect.
While mutations in the DHH gene are known to cause gonadal dysgenesis, we conclude that milder mutations/polymorphisms in this gene may result in male infertility without affecting testicular development or differentiation of the external genitalia. We observed four novel mutations in the DHH gene in the cases with different reproductive anomalies. In a case of complete gonadal dysgenesis, two mutations, insertion of G at c.1156insG (p. Arg385fs) and c.997A>G (p. Thr333Ala) substitution were observed, an insertion of G at c.1156insG (p. Arg385fs) position in exon 3 was observed in a case with bilateral undescended testis and azoospermia. A synonymous substitution, c. 543C>T (p. His181His) showed a significant correlation with male infertility, and another synonymous substitution, c.990G>A (p. Ala330Ala) was observed in an infertile patient with unilateral undescended testis. Mutations in the DHH gene have been previously reported in gonadal dysgenesis cases, but this is the first study on DHH mutations/polymorphisms in male infertility. While new mutations were identified in this study, the major limitation of the study is the lack of functional assays to affirm the causative nature of these mutations.
Acknowledgements
The authors are thankful to the participants and their families. The authors would like to thank the Council of Scientific and Industrial Research (CSIR) for funding under network scheme of projects (BSC0101). PM is thankful to the University Grants Commission (UGC) for the financial support (Ref no. 460/CSIR-UGC NET DEC.2017).
Funding
The study was financially supported by the Council of Scientific and Industrial Research (CSIR) under network scheme of projects (BSC0101).
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Human Ethics Committee (IHEC) of the Central Drug Research Institute, Lucknow. Informed consent was obtained from all the individual participants included in the study.
Consent for publication
The authors have generated the data and provided their consent for its publication.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rassoulzadegan M, Paquis-Flucklinger V, Bertino B, Sage J, Jasin M, Miyagawa K, van Heyningen V, Besmer P, Cuzin F. Transmeiotic differentiation of male germ cells in culture. Cell. 1993;75:997–1006. doi: 10.1016/0092-8674(93)90543-Y. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Q, Wang M, Yuan Y, Wang X, Fu R, Wan H, Xie M, Liu M, Guo X, Zheng Y, Feng G. Complete meiosis from embryonic stem cell-derived germ cells in vitro. Cell Stem Cell. 2016;18:330–340. doi: 10.1016/j.stem.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- 4.Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol. 1996;6:298–304. doi: 10.1016/S0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- 5.Szczepny A, Hime GR, Loveland KL. Expression of hedgehog signalling components in adult mouse testis. Dev Dyn. 2006;11:3063–3070. doi: 10.1002/dvdy.20931. [DOI] [PubMed] [Google Scholar]
- 6.Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod. 2000;63:1825–1838. doi: 10.1095/biolreprod63.6.1825. [DOI] [PubMed] [Google Scholar]
- 7.Yao HH, Whoriskey W, Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002;16:1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morales CR, Fox A, El-Alfy M, Ni X, Argraves WS. Expression of patched-1 and smoothened in testicular meiotic and post-meiotic cells. Microsc Res Tech. 2009;72:809–815. doi: 10.1002/jemt.20733. [DOI] [PubMed] [Google Scholar]
- 9.Mäkelä JA, Saario V, Bourguiba-Hachemi S, Nurmio M, Jahnukainen K, Parvinen M, Toppari J. Hedgehog signalling promotes germ cell survival in the rat testis. Reproduction. 2011;142:711–721. doi: 10.1530/REP-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, Serman L. The role of the Hedgehog signaling pathway in cancer: a comprehensive review. Bosn J Basic Med Sci. 2018;18:8–20. doi: 10.17305/bjbms.2018.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tate G, Satoh H, Endo Y, Mitsuya T. Assignment1 of Desert Hedgehog (DHH) to human chromosome bands 12q12→ q13. 1 by in situ hybridization. Cytogenet Genome Res. 2000;8:93–94. doi: 10.1159/000015495. [DOI] [PubMed] [Google Scholar]
- 12.Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121:1603–1614. doi: 10.1242/dev.121.6.1603. [DOI] [PubMed] [Google Scholar]
- 13.Kroft TL, Patterson J, Won Yoon J, Doglio L, Walterhouse DO, Iannaccone PM, Goldberg E. GLI1 localization in the germinal epithelial cells alternates between cytoplasm and nucleus: upregulation in transgenic mice blocks spermatogenesis in pachytene. Biol Reprod. 2001;65:1663–1671. doi: 10.1095/biolreprod65.6.1663. [DOI] [PubMed] [Google Scholar]
- 14.Umehara F, Tate G, Itoh K, Yamaguchi N, Douchi T, Mitsuya T, Osame M. A novel mutation of desert hedgehog in a patient with 46, XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am J Hum Genet. 2000;67:1302–1305. doi: 10.1086/321210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugie K, Futamura N, Suzumura A, Tate G, Umehara F. Hereditary motor and sensory neuropathy with minifascicle formation in a patient with 46XY pure gonadal dysgenesis: a new clinical entity. Ann Neurol. 2002;51:385–388. doi: 10.1002/ana.10150. [DOI] [PubMed] [Google Scholar]
- 16.Canto P, Söderlund D, Reyes E, Mendez JP. Mutations in the desert hedgehog (DHH) gene in patients with 46, XY complete pure gonadal dysgenesis. J Clin Endocrinol Metab. 2004;89:4480–4483. doi: 10.1210/jc.2004-0863. [DOI] [PubMed] [Google Scholar]
- 17.Canto P, Vilchis F, Söderlund D, Reyes E, Mendez JP. A heterozygous mutation in the desert hedgehog gene in patients with mixed gonadal dysgenesis. Mol Hum Reprod. 2005;11:833–836. doi: 10.1093/molehr/gah216. [DOI] [PubMed] [Google Scholar]
- 18.Das DK, Sanghavi D, Gawde H, IdiculaThomas S, Vasudevan L. Novel homozygous mutations in Desert hedgehog gene in patients with 46,XY complete gonadal dysgenesis and prediction of its structural and functional implications by computational methods. Eur J Med Genet. 2011;54:e529–e534. doi: 10.1016/j.ejmg.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Werner R, Merz H, Birnbaum W, Marshall L, Schröder T, Reiz B, Kavran JM, Bäumer T, Capetian P, Hiort O. 46,XY Gonadal dysgenesis due to a homozygous mutation in desert hedgehog (DHH) identified by exome sequencing. J Clin Endocrinol Metab. 2015;100:E1022–E1029. doi: 10.1210/jc.2015-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baldinotti F, Cavallaro T, Dati E, Baroncelli GI, Bertini V, Valetto A, Massart F, Fabrizi GM, Zanette G, Peroni D, Bertelloni S. Novel Familial variant of the desert hedgehog gene: clinical findings in two sisters with 46,XY gonadal dysgenesis or 46,XX karyotype and literature review. Horm Res Paediatr. 2018;89:141–149. doi: 10.1159/000485507. [DOI] [PubMed] [Google Scholar]
- 21.Paris F, Flatters D, Caburet S, Legois B, Servant N, Lefebvre H, et al. A novel variant of DHH in a familial case of 46,XY disorder of sex development: insights from molecular dynamics simulations. Clin Endocrinol. 2017;87:539–544. doi: 10.1111/cen.13420. [DOI] [PubMed] [Google Scholar]
- 22.Sato NS, Maekawa R, Ishiura H, Mitsui J, Naruse H, Tokushige SI, et al. Partial duplication of DHH causes minifascicular neuropathy: a novel mutation detection of DHH. Ann Clin Transl Neurol. 2017;4:415–421. doi: 10.1002/acn3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothacker KM, Ayers KL, Tang D, Joshi K, Van Den Bergen JA, Robevska G, Samnakay N, Nagarajan L, Francis K, Sinclair AH. Choong CS. A novel, homozygous mutation in desert hedgehog (DHH) in a 46, XY patient with dysgenetic testes presenting with primary amenorrhoea: a case report. Int J Pediatr Endocrinol. 2018;2018:2. doi: 10.1186/s13633-018-0056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su Z, Pan L, Wang L, Chen W, Song J, Li S. 46, XY Gonadal dysgenesis accompanied by neuropathy caused by a DHH mutation. In57th Annual ESPE 2018 Aug 28 (Vol. 89). European Society for Paediatric Endocrinology
- 25.Neocleous V, Fanis P, Cinarli F, Kokotsis V, Oulas A, Toumba M, Spyrou GM, Phylactou LA, Skordis N. 46, XY complete gonadal dysgenesis in a familial case with a rare mutation in the desert hedgehog (DHH) gene. Hormones. 2019;18:315–320. doi: 10.1007/s42000-019-00116-6. [DOI] [PubMed] [Google Scholar]
- 26.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 27.Howe B, Umrigar A, Tsien F. Chromosome preparation from cultured cells. J Vis Exp. 2014;83:e50203. doi: 10.3791/50203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008:pdb.prot4986. [DOI] [PubMed]
- 29.Simoni M, Bakker E, Krausz C. EAA/EMQN best practice guidelines for molecular diagnosis of y-chromosomal microdeletions. State of the art 2004. Int J Androl. 2004;27:240–249. doi: 10.1111/j.1365-2605.2004.00495.x. [DOI] [PubMed] [Google Scholar]
- 30.Thangaraj K, Singh L, Reddy AG, Rao VR, Sehgal SC, Underhill PA, Pierson M, Frame IG, Hagelberg E. Genetic affinities of the Andaman Islanders, a vanishing human population. Curr Biol. 2003;13:86–93. doi: 10.1016/S0960-9822(02)01336-2. [DOI] [PubMed] [Google Scholar]
- 31.Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Maixner W, Diatchenko L. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- 32.Sauna ZE, Kimchi-Sarfaty C. Understanding the contribution of synonymous mutations to human disease. Nat Rev Genet. 2011;12:683–691. doi: 10.1038/nrg3051. [DOI] [PubMed] [Google Scholar]