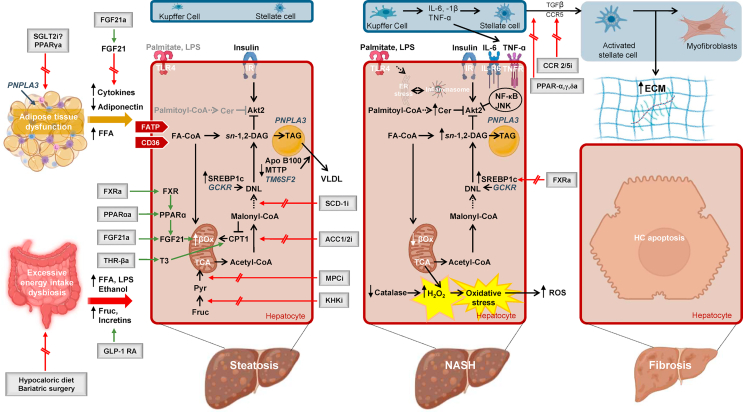
Figure 1.
Pathogenetic mechanisms driving the progression of human NAFLD and key pathways of therapeutic interventions. High caloric intake induces changes in the gut microbiota and enlargement of the adipose tissue. Diet and bariatric surgery are the main treatment strategies in this context. Gut dysbiosis is associated with disruption of intercellular tight junctions [87], which permit the translocation of bacterial lipopolysaccharide (LPS) into the systemic circulation and increase alcohol-producing bacteria [85]. The patatin-like phospholipase domain-containing protein 3 (PNPLA3) genetic variant increases adipose tissue lipolysis [17], increasing the flux of substrates and signaling molecules to the liver [92]. Adipose tissue–liver cross talk is further mediated through the secretion of exosomes [97] and adipokines [94]. Steatotic hepatocytes are characterized by a potentially upregulated uptake of FFA. Additionally, enhanced DNL converts acetyl-CoA to new fatty acids [109,112]. The genetic variant of the gene glucokinase regulatory protein (GCKR) contributes to elevated DNL, through increase in substrates availability [43]. In this context, mitochondrial fatty acid oxidation is upregulated [121]. Export of triglycerides as VLDL particles is compromised through reduced lipidation of microsomal triglyceride transfer protein (MTTP), the enzyme that catalyzes the lipidation of apolipoprotein B100 (apoB100) [138]. The transmembrane 6 superfamily member 2 (TM6SF2) genetic variant further attenuates the ability of hepatocytes to mobilize neutral lipids for the VLDL assembly [38]. Mitochondrial pyruvate carrier inhibitors (MPCi) decrease carbon flow into the tricarboxylic acid (TCA) cycle and therefore alter the ability of hepatic mitochondria to fuel DNL. A number of further pharmacological agents including fibroblast growth factor agonists (FGF21a), peroxisome proliferator-activated receptor (PPAR) ɑ agonists (PPARαa), thyroid hormone receptor-β agonists (THR-βa), and farnesoid X receptor agonists (FXRa) mainly aim to augment fatty acid oxidation, and DNL is targeted by ketohexokinase inhibitors (KHKi), stearoyl-CoA desaturase 1 inhibitors (SCD-1i), mitochondrial pyruvate carrier inhibitors (MPCi), and FXRa and acetyl coenzyme A carboxylase inhibitors (ACC1/2i). Following progression to NASH, production of sn-1,2-DAG and sphingolipids is favored. In NASH, the sn-1,2-DAG–PKCε pathway tightly correlates with hepatic insulin resistance [115]; hepatic dihydroceramides correlate with hepatic oxidative stress and inflammation [118]. Following progression to NASH, mitochondrial flexibility is lost, leading to decreased fatty acid oxidation [121] and oxidative stress and then inflammation [118], leading to hepatocytes apoptosis. Resident macrophage cells in the liver, the Kupffer cells, are increased and release proinflammatory cytokines [143]. Finally, HSC activation is regarded as a key initiating event in hepatic fibrogenesis, with activated HSC being characterized by enhanced extracellular matrix (ECM) production. Among other factors, the transforming growth factor β (TGF- β) initiates ECM gene expression in quiescent HSC [148]. Chemokine receptor (CCR2/5) antagonists and pan-PPARa mainly exert antifibrotic properties. ACC 1/2i, acetyl coenzyme A carboxylase 1/2 inhibitors; a: agonists, apoB100, apolipoprotein B100; βox, β oxidation; CER, ceramides; ChREBP, carbohydrate regulatory element binding protein; CCR, chemokine receptor; CD36, Cluster of differentiation 36; CPT1, carnitine palmitoyltransferase 1; DAG, diacylglycerols; DNL, de novo lipogenesis; ER, endoplasmic reticulum; ECM, extracellular matrix; Fa-CoA, fatty acyl-CoA; FATP, fatty acid transporters; FGF, fibroblast growth factor; FFA, free fatty acids; fruc, fructose; FXR, farnesoid X receptor; GCKR, glucokinase regulatory protein; GLP-1 RA, glucagon-like peptide 1 receptor agonists (GLP-1 RA); HC, hepatocyte; HSCs, hepatic stellate cells; i, inhibitors; IL-1β, interleukin 1β; IL-6, interleukin 6; IL-6R, interleukin 6 receptro; IR, insulin receptor; JNK, c-Jun N-terminal kinase; KHKi, ketohexokinase inhibitors; LD, lipid droplet; LPS, lipopolysaccharide; MPCi, mitochondrial pyruvate carrier inhibitors; MTTP, microsomal triglyceride transfer protein; NF-κB: nuclear factor κ-light-chain-enhancer of activated B cells; PNPLA3, patatin-like phospholipase domain-containing protein 3; PPAR, peroxisome proliferator-activated receptor; pyr, pyruvate, SCD-1i, stearoyl-CoA desaturase 1 inhibitors; SGLT-2i, sodium glucose cotransporter-2 inhibitors; SREBP1c, sterol regulatory element binding protein 1c; TAG, triacylglycerol; TCA, tricarboxylic acid; TGF-β, transforming growth factor-β; THR, thyroid hormone receptor; TLR-4, toll-like receptor-4; TNF-α, tumor necrosis factor-α; TNFR, tumor necrosis factor receptor; TM6SF2, transmembrane 6 superfamily member 2; VLDL, very low density lipoprotein.