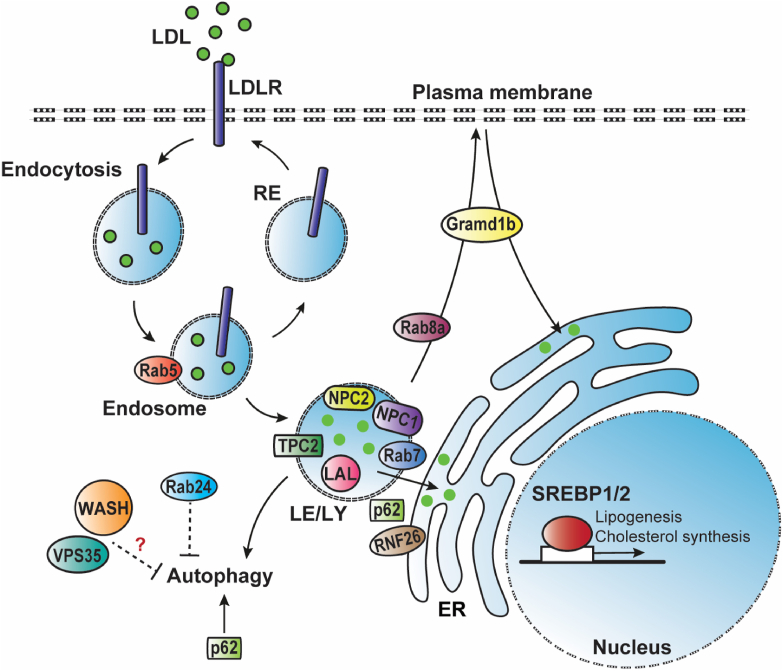
Figure 1.
Cholesterol transport through the endolysosomal network. After binding to LDLR, LDL-cholesterol is internalized, and the LDL-LDLR complex is transported to endosomes, where LDL-cholesterol dissociates from LDLR. LDLR can be transported back to the plasma membrane via recycling endosomes (RE). LDL-cholesterol is transported to late endosome/lysosome (LE/LY), where lysosomal acid lipase (LAL) hydrolyzes cholesterol esters to release free cholesterol. TPC2 is important for LE-LY fusion and thus for further cholesterol transport. NPC2 can bind and deliver free cholesterol to NPC1. NPC1 regulates cholesterol transport to other cellular compartments, such as the endoplasmic reticulum (ER) by interacting with Rab7 and the plasma membrane via Rab8a. The ER-located RNF26 recruits and ubiquitinates p62, leading to the binding of specific endosomal-associated adaptor proteins to mediate the positioning of vesicles in the perinuclear area, but its function in cholesterol transport needs to be elucidated. Gramd1b, or Aster-B, is implicated in both LE/LY-to-PM and PM-to-ER cholesterol transport. When cholesterol levels in the ER are low, SREBP is translocated to the nucleus, where it induces transcription of genes involved in lipogenesis and cholesterol uptake and synthesis. Cholesterol levels in LE/LY affect lysosomal function and thereby also the autophagic pathway, in which p62 is an important player. Rab24 is thought to reduce autophagy and thereby contribute to MAFLD. The endosomal WASH complex and the retromer subunit VPS35 are also known to affect autophagy, but their physiological role in this context and in MAFLD remain unknown.