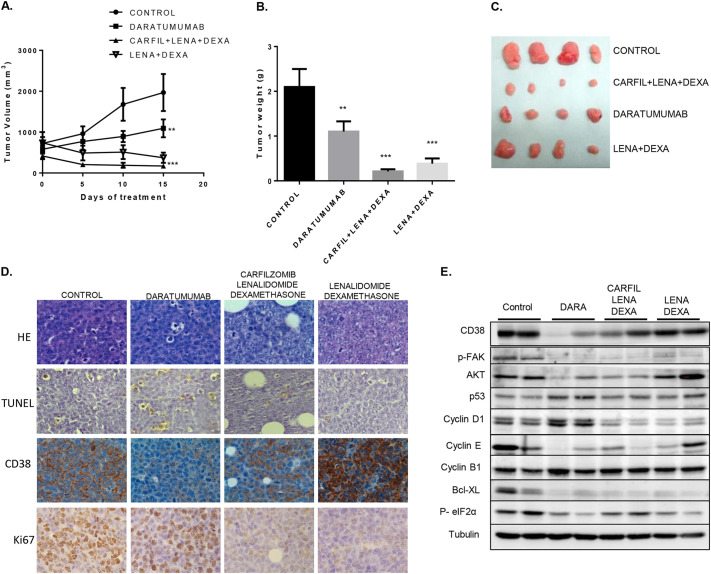
Fig. 7.
In vivo therapeutic assays using EMM PDOX. EMM xenografts were expanded orthotopically to 40 additional NSG mice. After evidence of homogeneous tumor growth, animals were randomly distributed in four groups (n=10) and treated with saline (control), with daratumumab (DARA) in monotherapy, with the combination of lenalidomide (LENA) and dexamethasone (DEXA), or the combination of lenalidomide, dexamethasone and carfilzomib (CARFIL) for 15 days. No signs of animal toxicity were observed with any of these treatments. (A) Evaluation of tumor volumes during tumor treatment. (B) Tumor weights at the end of the treatment at mice sacrifice for the different experimental groups. (C) Representative diagram of tumors dissected at sacrifice for the different experimental groups. (D) H&E staining, IHQ evaluation of Ki67 and CD38, and TUNEL assay (at 400× magnification). A decrease of CD38 expression was observed only in daratumumab-treated tumors. A decrease of Ki67 was observed after the treatment with lenalidomide-dexamethasone with or without carfilzomib, without a significant increase of apoptotic cells. A pro-apoptotic effect was observed in daratumumab tumors with a significant increase of apoptotic cells evaluated by TUNEL assay with respect to the control group without significant Ki67 expression change. (E) Western blot analysis of CD38 and the relevant proteins implicated in cell cycle control and apoptosis. A decrease of CD38 expression was observed also by western blot only in daratumumab-treated tumors. Data are mean±s.e.m.