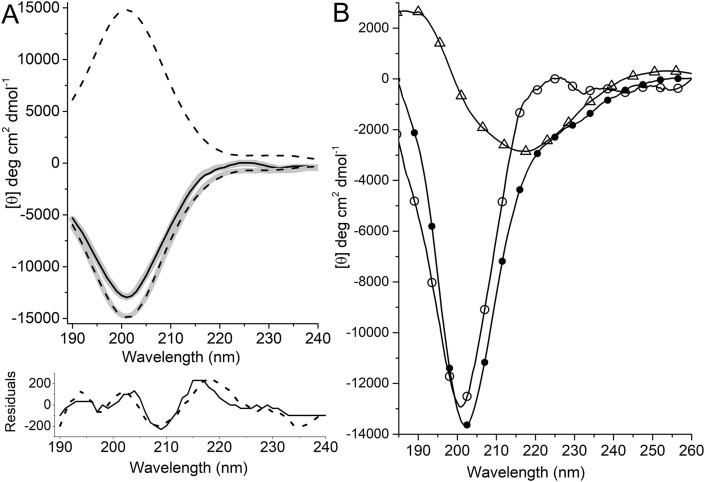
Fig. 3.
A) Far-UV CD spectra of the peptide EQRPR and derived curves calculated by CDSSTR method. Positive and negative sign dashed black curves represent the peptides with all d-isomer and its mirror-image, respectively. Solid black line is CD spectrum of all l-isomer peptide. CDSSTR derived curves of the peptides conform well to the respective experimental spectra. B) Secondary structure transition in the peptide EQRPR induced by TFE. Far-UV CD spectra of the peptide in buffer pH 7.3 (open cirles), in 50% (v/v) TFE (solid circles) and their difference (open triangles). The minimum observed around 218 nm in the difference spectrum indicates beta-sheet formation.