Fig. 1.
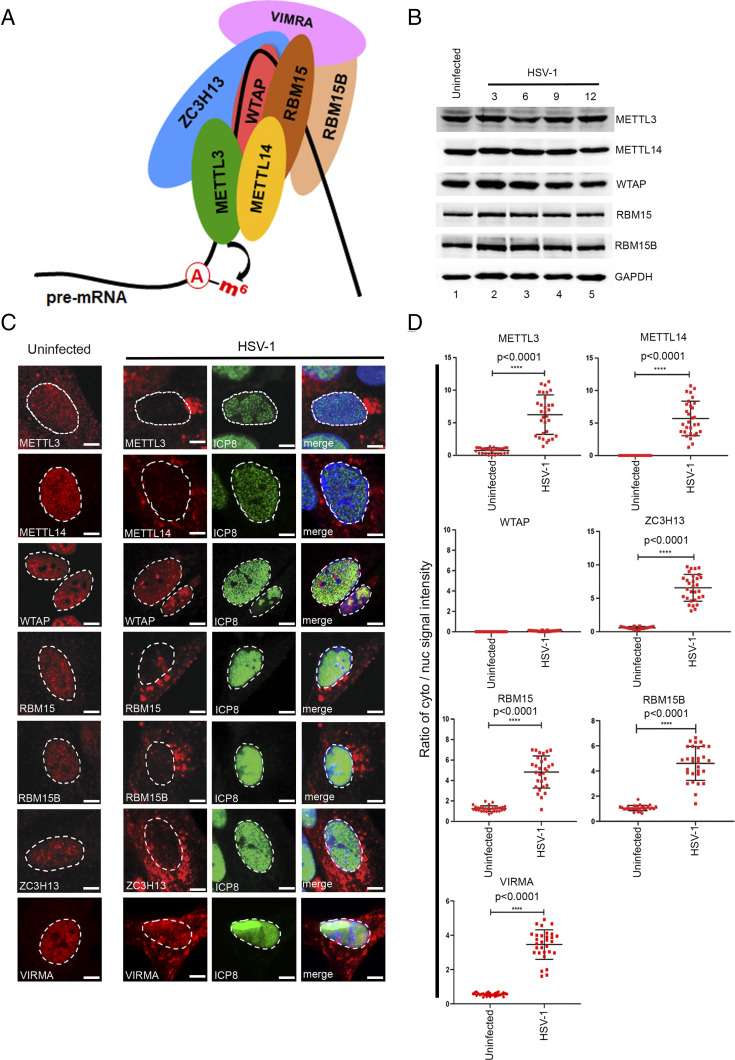
HSV-1 infection leads to a dramatic redistribution of the nuclear m6A methyltransferase subunits. (A) Schematic showing the principal subunits of the m6A methyltransferase complex. Catalysis is provided by METTL3, which forms a stable heterodimer with METTL14. Association with WTAP is required for localization of METTL3/METTL14 to nuclear speckles. Additional subunits ZC3H13, VIRMA, and RBM15/15B are thought to confer substrate specificity. Adenosine residues are methylated during pre-mRNA synthesis prior to release of the RNA (thick black line) from the DNA template. (B) Immunoblots showing the abundance of METTL3, METTL14, WTAP, RBM15, and RMB15B over a time course of HSV-1 (strain KOS) infection. GAPDH provides a loading control. (C) Indirect immunofluorescence detection of the core (METTL3, METTL14, and WTAP) and accessory (RBM15, RBM15B, and ZC3H13) methyltransferase complex subunits in NHDFs after 12 h of HSV-1 (strain KOS) infection. DAPI was used to define the boundary between the nucleus and cytoplasm as indicated with a dashed line. (Scale bar, 10 µm.) (D) Quantification of the ratio of the cytoplasmic/nuclear signal using ImageJ from 30 different images from each condition. Statistical significance was determined using Student’s t test. The mean ± SEM are shown along with the P value for the difference between uninfected and infected cultures.