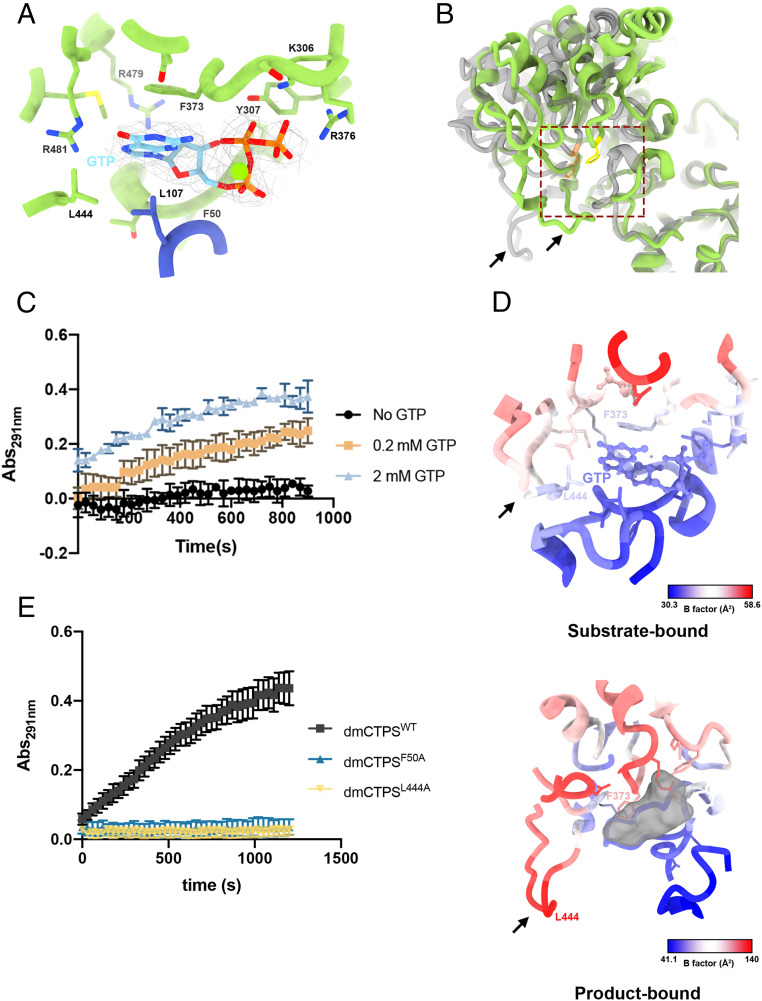
Fig. 3.
Determination of GTP binding site and relevant conformational changes reveal the mechanism of allosteric regulation. (A) The GTP binding site at GAT domain. Residues interacting with GTP are indicated. (B) The structure comparison of the GTP binding sites in dmCTPS+Sub (green) and dmCTPS+Pro (gray) models shows the general conformational change of the GAT domain and the switch of the wing region. Phe373 is shown in yellow and orange in dmCTPS+Sub and dmCTPS+Pro models, respectively. The wing structure is indicated by arrows. The AL domain (1 to 280) was used for the alignment. (C) Analysis for generation of CTP by wild-type dmCTPS (dmCTPSWT) in conditions with 0, 0.2, and 2 mM GTP. The absorption of 291 nm (Abs291nm) represents the concentration of CTP in samples. For the analysis, 2 μM dmCTPS was mixed with 2 mM UTP, 2mM ATP, 10 mM MgCl2, 25 mM Tris HCl (pH 7.5), and GTP at different concentrations before the supplement of 10 mM glutamine for initiating the reaction. (Error bars, SD.) (D) B factors are shown on GTP binding sites in dmCTPS+Sub and dmCTPS+Pro models. Arrows indicate the wing structure. (E) Analysis for generation of CTP by mutant dmCTPS (F50A and L444A) in condition with 0.2 mM GTP.