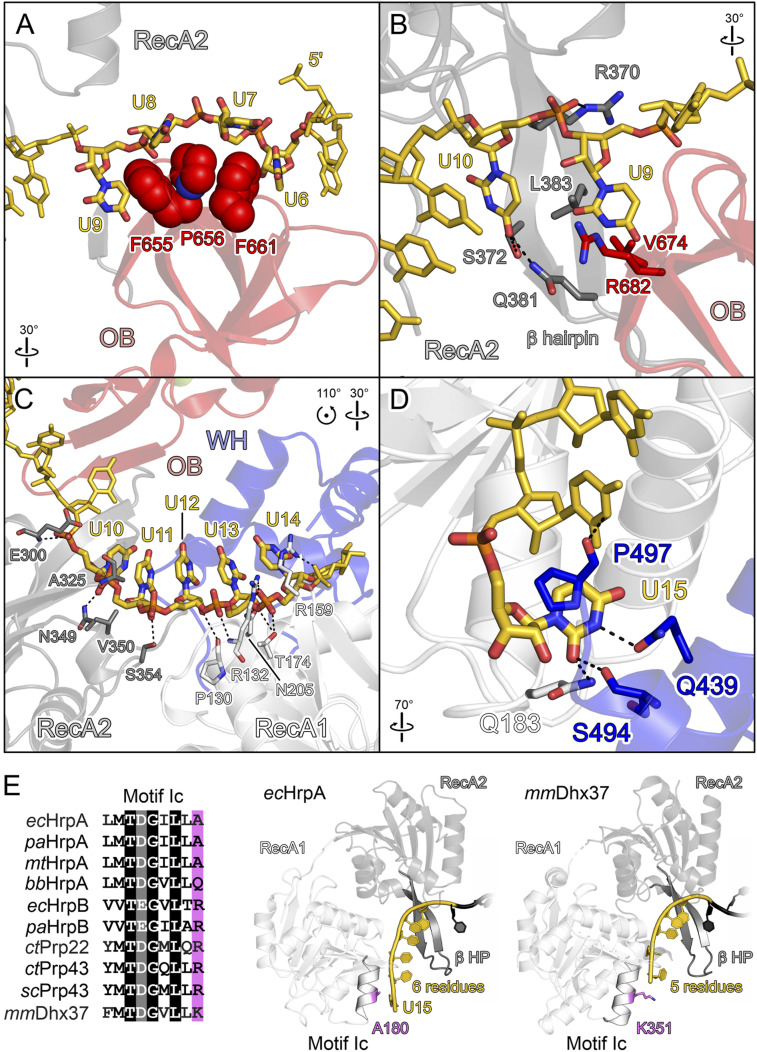
Fig. 4.
RNA binding by ecHrpA1-783. (A–D) Close-up views of ecHrpA1-783 residues that contact RNA from the 5′ to the 3′ end. Protein regions are shown as semitransparent cartoons. Interacting protein and RNA residues are highlighted as spheres or sticks and color-coded by atom type. In this and the following figures: carbon, as the respective protein/RNA domain/region; nitrogen, blue; oxygen, red; phosphorus, orange. Dashed black lines, hydrogen bonds or salt bridges. (A) RNA residues U6 to U9 wrapping around the OB stacking triad (F655, P656, and F661). (B) Nucleotides U9 and U10 binding across the continuous β-sheet formed between the prominent RecA2 β-hairpin and the OB domain. (C) RNA residues U10 to U14 binding across the RecA1/2 RNA binding surfaces. (D) Nucleotide U15 bound at an ecHrpA-specific pocket formed from residues of the RecA1 and WH domains. (E) Multiple sequence alignment (Left) and RNA binding (Right) to motif Ic in bacterial and eukaryotic DEAH/RHA proteins; ec, E. coli; pa, P. aeruginosa; mt, Mycobacterium tuberculosis; bb, B. burgdorferi; ct, C. thermophilum; sc, S. cerevisiae; mm, M. musculus. In contrast to other DEAH/RHA proteins, the small alanine at the C terminus of motic Ic in the majority of HrpA orthologs allows binding of an additional RNA nucleobase (U15). Divergent C-terminal motif Ic residues, violet.