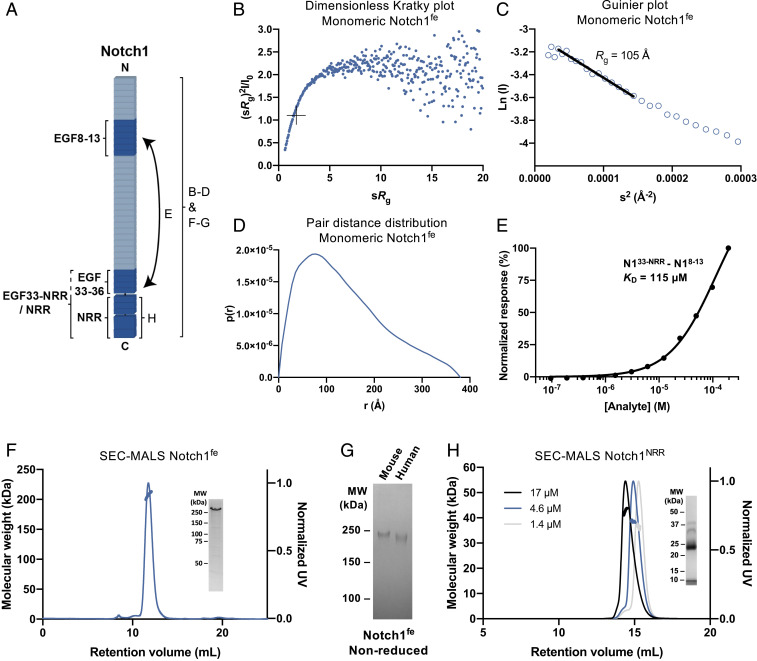
Fig. 3.
Notch1fe is flexible and the NRR dimerizes weakly. (A) Schematic representation of the interaction and biophysical experiments on regions reported in B–H. (B–D) Structural analysis of monomeric Notch1fe from SEC-SAXS, including the Dimensionless Kratky plot with crosshairs indicating the peak position for a globular protein (B), Guinier plot with a black line indicating the fit used to derive the Rg (C), and pair distance distribution function (D). (E) SPR equilibrium-binding plot of Notch1EGF33–NRR to Notch1EGF8–13. (F) SEC-MALS analysis of Notch1fe shows a monomeric and monodisperse sample (thick lines indicate the molecular weight, left axis). (Inset) Coomassie-stained SDS-PAGE of purified Notch1fe in reducing conditions. UV, ultraviolet. (G) Coomassie-stained SDS-PAGE of purified Notch1fe in nonreducing conditions. MW, molecular weight. (H) SEC-MALS analysis of Notch1NRR at three concentrations, determined at elution, shows a monomer–dimer equilibrium (thick lines indicate the molecular weight, left axis). (Inset) Coomassie-stained SDS-PAGE of purified Notch1NRR in reducing conditions, note that Notch1NRR is processed at the S1 cleavage site into two fragments of 8 and 27 kDa.