Abstract
Background
As the COVID-19 pandemic moves into the survivorship phase, questions regarding long-term lung damage remain unanswered. Previous histopathologic studies are limited to autopsy reports. We studied lung specimens from COVID-19 survivors who underwent elective lung resections to determine whether postacute histopathologic changes are present.
Methods
This multicenter observational study included 11 adult COVID-19 survivors who had recovered but subsequently underwent unrelated elective lung resection for indeterminate lung nodules or lung cancer. We compared these against an age- and procedure-matched control group who never contracted COVID-19 (n = 5) and an end-stage COVID-19 group (n = 3). A blinded pulmonary pathologist examined the lung parenchyma focusing on 4 compartments: airways, alveoli, interstitium, and vasculature.
Results
Elective lung resection was performed in 11 COVID-19 survivors with asymptomatic (n = 4), moderate (n = 4), and severe (n = 3) COVID-19 infections at a median 68.5 days (range 24-142 days) after the COVID-19 diagnosis. The most common operation was lobectomy (75%). Histopathologic examination identified no differences between the lung parenchyma of COVID-19 survivors and controls across all compartments examined. Conversely, patients in the end-stage COVID-19 group showed fibrotic diffuse alveolar damage with intra-alveolar macrophages, organizing pneumonia, and focal interstitial emphysema.
Conclusions
In this study to examine the lung parenchyma of COVID-19 survivors, we did not find distinct postacute histopathologic changes to suggest permanent pulmonary damage. These results are reassuring for COVID-19 survivors who recover and become asymptomatic.
Dr Donington discloses a financial relationship with AstraZeneca, BMS, and Roche/Genantech.
One year into the COVID-19 pandemic, more than 116 million people worldwide have been infected with SARS-CoV-2 and more than 2.5 million lives have been lost.1 Postmortem autopsies or pathologic studies from patients with end-stage lung disease from COVID-19 report severe fibrosis, diffuse alveolar damage (DAD), perivascular T-cell infiltration, severe endothelial injury, intracellular viral particles, and cell membrane disruption in lung tissue.2, 3, 4 Whether there are long-lasting histopathologic changes in the lung parenchyma of COVID-19 survivors is unknown.5 , 6
A growing number of people have survived COVID-19, but some have experienced a prolonged and strenuous recovery. In population-based studies, an estimated 35% of survivors had not returned to their usual state of health 14 to 21 days postinfection, and some patients remained symptomatic several months after infection,7, 8, 9, 10 even showing persistent radiographic findings such as ground-glass opacities.5 Although a lung biopsy is unlikely to be performed on an otherwise healthy survivor, there is a unique opportunity to examine the lung parenchyma of COVID-19 survivors who subsequently underwent elective thoracic operation or lung resection for different indications such as solitary lung nodules or lung cancer. Examining the lung parenchyma of this patient population can provide important insights into this disease and its long-term pulmonary impact.
In this context, we studied the histopathologic changes in a unique subset of patients who recovered from COVID-19, became asymptomatic, and underwent lung resection for other unrelated indications. We sought to identify the spectrum of disease in COVID-19 survivors, which would help inform expectations in a growing surviving population currently exceeding millions.
Patients and Methods
Study Design and Participants
This multicenter, observational, retrospective, cohort study was conducted at Loyola University Medical Center and the University of Chicago Medicine. The study was approved by the Loyola University Chicago and the University of Chicago Institutional Review Boards (LU214441 and IRB210055).
We included 11 adult patients (>18 years) who had previously contracted COVID-19 confirmed by a positive reverse-transcription polymerase chain reaction and were asymptomatic at the time of their subsequent elective lung resections between July 2020 and February 2021 (COVID-19 survivors). For comparison, we also included 3 patients who had prolonged symptoms and did not fully recover from COVID-19 (COVID-19 end-stage lung disease). The negative control group included 5 patients matched for age and elective thoracic procedure who underwent lung resection between 2019 and 2020 and did not have a history of COVID-19. Patient clinical, histologic, and radiologic data were collected from electronic medical records.
COVID-19 Disease
Details of the COVID-19 course, including date of positive reverse-transcription polymerase chain reaction test result, symptoms, and hospital and intensive care unit (ICU) admissions, were included. COVID-19 survivors were classified by their symptoms during infection into 3 groups: asymptomatic, moderate, or severe based on prior literature.11, 12, 13, 14, 15 Asymptomatic patients did not experience symptoms of COVID-19.11 , 12 Moderate COVID-19 cases were defined by symptoms such as fever, chills, aches, cough, headache, nausea, sore throat, fatigue, and congestion not requiring ICU admission.12, 13, 14, 15 Severe disease was determined by admission to the ICU.15
Pathologic Review
All specimens were handled according to routine institutional histopathology processing protocols. Sections of uninvolved lung parenchyma distant from the main tumor were taken at the time of grossing for each of the cancer resection specimens. For the explanted lung specimen, representative sections were taken from the peripheral, central, and hilar regions of each lobe. In brief, 5-μm, formalin-fixed paraffin-embedded sections were stained with hematoxylin and eosin. Trichrome stains were also performed on representative sections of the explanted lung. Stained sections were reviewed by board-certified pathologists (V.A. and P.M.) experienced in thoracic pathology. Representative photos were taken at ×2.5, ×10, and ×20 magnifications.
Randomized and deidentified slides were provided to a blinded, experienced thoracic pathologist (A.N.H.) to assess whether any specific pathologic changes could be seen in the COVID-19 survivors compared with the negative controls. Representative slides from the patients with COVID-19 end-stage lung disease were used as comparators to demonstrate to the blinded pathologist both acute and chronic changes directly attributable to COVID-19. Histologic features were assessed in a standardized fashion across 4 histologic compartments: airways, alveoli, interstitium, and vasculature.
Statistical Analysis
Results are descriptively reported with percentages or medians with ranges as appropriate.
Results
Patient Characteristics
COVID-19 survivors
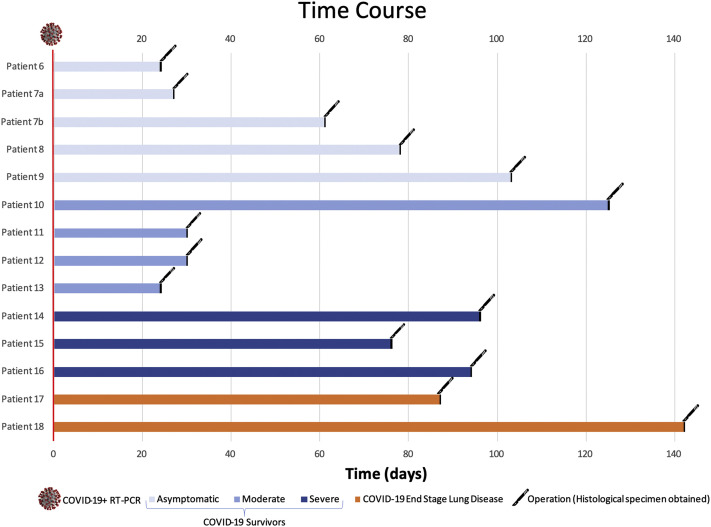
Among COVID-19 survivors, median age was 65 years (range, 36-72 years), median body mass index was 27.9 kg/m2 (range, 23.8-34.7 kg/m2), and 7 (64%) were women. COVID 19 was moderate in 4 survivors (36%), 3 (27%) had a severe course requiring ICU admission, and the remainder (36%) were asymptomatic. Common preexisting conditions included hypertension (36%), obesity (27%), and heart disease (18%) (Table 1 ). Although no COVID-19 survivors were current smokers, 82% were former smokers. At time of operation, all patients had resolution of COVID-19 radiographic sequelae (Figure 1 ) and all were asymptomatic from a COVID-19 perspective.
Table 1.
Summary of Demographic Data, Clinical Characteristics, COVID-19 Course, and Operative Details of 14 Patients
| Characteristics | COVID-19 survivors |
COVID-19 end-stage lung disease |
Negative control patients |
|---|---|---|---|
| (n = 11) | (n = 3 | (n = 5) | |
| Age, y | 65 (36-78) | 56 (53-77) | 72 (62-78) |
| Female sex | 7 (64) | 1 (33) | 3 (60) |
| Race/ethnic group | |||
| White | 7 (64) | 2 (67) | 3 (60) |
| African American | 2 (18) | NA | 2 (40) |
| Hispanic/Latino | 2 (18) | 1 (33) | NA |
| Body mass index, kg/m2 | 27.9 (23.8-34.7) | 21.9 (21.3-24) | 28.7 (24.6-34.7) |
| Smoking | |||
| None | 2 (18) | 2 (67) | NA |
| Current | NA | NA | NA |
| Former | 9 (82) | 1 (33) | 5 (100) |
| Pack-years | 25.5 (6-52.5) | 29.5 | 50 (18-67.5) |
| Comorbidities | |||
| Chronic obstructive pulmonary disease | 2 (18) | NA | NA |
| Chronic kidney disease | 1 (9) | 1 (33) | NA |
| Heart disease | 2 (18) | NA | NA |
| Hypertension | 4 (36) | 1 (33) | 2 (40) |
| Obesity | 3 (27) | NA | NA |
| Type 2 diabetes | 1 (9) | 1 (33) | NA |
| Othera | 4 (36) | 3 (100) | 4 (80) |
| COVID-19 symptoms | |||
| Anosmia | 1 (9) | NA | NA |
| Chills | 2 (18) | NA | NA |
| Congestion | 4 (36) | NA | NA |
| Cough | 6 (55) | 2 (67) | NA |
| Fatigue | 3 (27) | NA | NA |
| Fever | 4 (36) | 2 (67) | NA |
| Headache | 2 (18) | NA | NA |
| Muscle/body aches | 2 (18) | NA | NA |
| Nausea | 1 (9) | NA | NA |
| Pneumonia | 2 (18) | 3 (100) | NA |
| Shortness of breath | 1 (9) | 2 (67) | NA |
| Sore throat | 3 (27) | NA | NA |
| Classification of COVID-19 course | |||
| Asymptomatic | 4 (36) | NA | NA |
| Moderate | 4 (36) | NA | NA |
| Severe | 3 (27) | 3 (100) | NA |
| Days from infection to thoracic procedure or deathb | 68.5 (24-125), n = 12 | 87 (87-142) | NA |
Continuous data are presented as median (range) and categorical data as n (%).
NA, not applicable.
Other comorbidities included obstructive sleep apnea, Crohn disease, hyperthyroidism, hyperlipidemia, and hepatitis C.
One patient underwent 2 elective thoracic surgeries, and those 2 specimens (n = 12 total specimens) were included.
Figure 1.
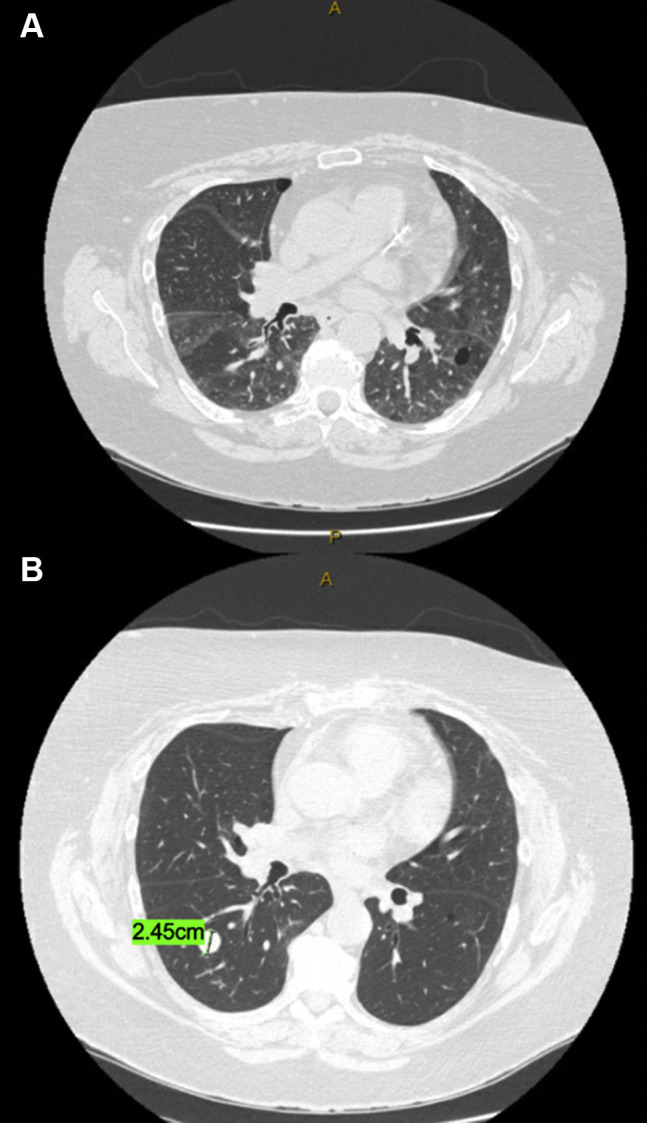
Representative computed tomographic (CT) images from a patient who recovered from severe COVID-19 infection and then subsequently underwent a right lower lobectomy. (A) Axial CT images 3 weeks after the COVID-19 diagnosis show bilateral patchy ground-glass opacities. (B) Axial CT from positron emission tomography/CT images 6 weeks later show resolution of the bilateral ground-glass opacities and the 2.45-cm lung nodule prompting the right lower lobectomy.
COVID-19 end-stage lung disease patients
Included were 3 patients with COVID-19 end-stage lung disease: a 56-year-old previously healthy man (patient 17), a 53-year-old man (patient 18) with type 2 diabetes, hyperlipidemia, and hypertension, and a 77-year-old woman (patient 19) with chronic kidney disease and chronic lymphocytic leukemia (Table 2 ).
Table 2.
Detailed Demographics of All 19 Patients
| Patients | Age, y | Sex | Characterization of COVID-19 Course | Type of Thoracic Procedure | Reason for Procedure |
|---|---|---|---|---|---|
| Negative control patients | |||||
| 1 | 78 | Female | NA | Wedge resection | Indeterminate nodule |
| 2 | 62 | Female | NA | Right lower lobectomy | Malignant neoplasm |
| 3 | 78 | Male | NA | Wedge resection | Indeterminate nodule |
| 4 | 72 | Female | NA | Left upper lobectomy | Malignant neoplasm |
| 5 | 68 | Male | NA | Right middle lobectomy | Adenocarcinoma |
| COVID-19 survivors | |||||
| 6 | 67 | Male | Asymptomatic | Right middle lobectomy | Malignant neoplasm |
| 7a | 51 | Female | Asymptomatic | Left upper lobectomy; right upper lobectomy | Indeterminate nodule; indeterminate nodule |
| 8 | 36 | Male | Asymptomatic | Wedge resection and pleurodesis | Spontaneous pneumothorax |
| 9 | 68 | Male | Asymptomatic | Right upper lobectomy | Indeterminate nodule |
| 10 | 53 | Female | Moderate | Right upper lobectomy | Adenocarcinoma |
| 11 | 72 | Female | Moderate | Right upper lobectomy | Adenocarcinoma |
| 12 | 65 | Female | Moderate | Right lower lobectomy | Indeterminate nodule |
| 13 | 50 | Male | Moderate | Wedge resection | Indeterminate nodule |
| 14 | 65 | Female | Severe | Right upper lobectomy | |
| 15 | 72 | Female | Severe | Wedge resection | Indeterminant nodule |
| 16 | 69 | Female | Severe | Right lower lobectomy | Indeterminant nodule |
| COVID-19 end-stage lung disease | |||||
| 17 | 56 | Male | Severe | Thoracotomy, decortication, bullectomy | Empyema and persistent air leak |
| 18 | 53 | Male | Severe | Lung transplant | ARDS and pulmonary fibrosis |
| 19 | 77 | Female | Severe | NA | NA |
NA, not applicable.
Patient 7 underwent 2 different elective thoracic operation at different times and therefore added 2 histologic specimens to the totals of COVID-19 survivors (n = 11 patients, n = 12 specimens).
Negative control patients
Specimens from 5 patients matched by age and procedure without a prior COVID-19 diagnosis served as control tissues and were obtained from lung cancer resection specimens in a similar fashion to the COVID-19 survivors. The controls all had a history of smoking.
No patient received any COVID-19 vaccine at the time of this study.
Operative Details and Indications
COVID-19 survivors
A total of 12 operations were performed in 11 COVID-19 survivors, resulting in 12 specimens available for analysis. The median time from infection to elective thoracic operation was 68.5 days (range, 24-125 days) (Figure 2 ). The most common procedure was lobectomy (75%), followed by wedge resection (25%) (Table 2). The indications for surgical procedure included management of indeterminate pulmonary nodules (58%), resection of biopsy-proven lung cancer (33%), and spontaneous pneumothorax (8%). There were no postoperative complications in the COVID-19 survivors.
Figure 2.
Amount of time from COVID-19 infection to operation. Time from positive COVID-19 reverse-transcription polymerase chain reaction (RT-PCR) test to elective thoracic operation date for COVID-19 survivors and patients with COVID-19 end-stage lung disease, with the exception of patient 19 who did not undergo an operation.
COVID-19 end-stage lung disease patients
Patient 17 underwent a thoracotomy, decortication, and bullectomy for empyema and persistent air leak 87 days after infection and remained ventilator dependent. Patient 18 underwent a bilateral lung transplant 142 days after infection. There were no postoperative complications. Patient 19 died of acute COVID-19 without undergoing thoracic operation.
Negative control patients
All 5 control patients underwent elective thoracic operations for lung cancer, with the most common procedures being lobectomy (60%) and wedge resection (40%).
Histology
Airways
In COVID-19 survivors and controls, there was evidence of small-airway disease, including basement membrane fibrosis, which was present in 67% of COVID-19 survivors and in 60% of control patients (Table 3 ; Figures 3 A, 3B). Airway inflammation was minimal in COVID-19 survivors and controls (Figures 3A, 3B). Examination of the airways in the patient who died of acute COVID-19 was somewhat limited by postmortem changes, but overall, there was scant-to-mild lymphocytic inflammation and minimal changes to the basement membrane or smooth muscle (Figure 3C). In patient 17, there was evidence of basement membrane fibrosis and moderate mixed inflammation in the submucosa, with intraepithelial extension suggestive of potential superimposed infectious processes (Figure 3D).
Table 3.
Summary of Histologic Findings
| Patients | Airway | Alveoli | Interstitium | Vessel |
|---|---|---|---|---|
| Negative control patients | ||||
| 1 | Unremarkable | Emphysema, pigmented macrophages, focal organizing pneumonia | Mild interstitial fibrosis, carcinoid (micro)tumorlet | PH |
| 2 | Basement membrane fibrosis/small airway disease | Mild emphysema, bronchiolar metaplasia | Anthracosis, focal lymphoid aggregates, focal mild fibrosis | Unremarkable |
| 3 | Goblet cell hyperplasia | Emphysema, rare giant cells | Anthracosis | PH |
| 4 | Basement membrane fibrosis | Mild emphysema, edema, rare poorly formed granulomas with giant cells | Variably fibrotic with some severely fibrotic areas, foci of interstitial lymphoid infiltrate, anthracosis | PH, granuloma in vessel wall |
| 5 | Unremarkable | Mild emphysema | Anthracosis | Unremarkable |
| COVID-19 survivors | ||||
| 6 | Unremarkable | Emphysema, atypical adenomatous hyperplasia, rare pigmented macrophages | Anthracosis | Unremarkable |
| 7a | Respiratory bronchiolitis | Emphysema, pigmented macrophages, Langerhans cell histiocytosis | Smoking-related interstitial fibrosis, scattered lymphoid aggregates | Unremarkable |
| 7b | Respiratory bronchiolitis, peribronchiolar fibrosis | Emphysema, pigmented macrophages, focal edema | Smoking-related interstitial fibrosis | Unremarkable |
| 8 | Mild peribronchiolar inflammation | Emphysema, focal poorly formed granulomas | Mild interstitial thickening, perivascular lymphoid aggregates | Unremarkable |
| 9 | Peribronchiolar fibrosis | Emphysema, with patchy chronic inflammation, focal edema | Patchy widened, smoking-related interstitial fibrosis | PH |
| 10 | Unremarkable | Mild emphysema | Mild interstitial fibrosis, anthracosis | PH |
| 11 | Basement membrane fibrosis | Emphysema, rare foamy macrophages | Rare foci of fibrosis, anthracosis | PH |
| 12 | Basement membrane fibrosis/small airway disease | Scattered poorly-formed granulomas and mild patchy inflammation | Poorly formed granulomas, anthracosis | PH |
| 13 | Mild small airway disease | Emphysema, scattered foamy macrophages | Unremarkable | PH |
| 14 | Unremarkable | Emphysema | Congestion, focal lymphoid infiltrate | Unremarkable |
| 15 | Peribronchial inflammation and fibrosis | Emphysema, rare organizing pneumonia | Mild interstitial inflammation, patchy fibrosis | Unremarkable |
| 16 | Unremarkable | Mild emphysema, edema, focal fibrin | Unremarkable | Unremarkable |
| COVID-19 end-stage lung disease | ||||
| 17 | Submucosal lymphohistiocytic infiltrate | Intra-alveolar macrophages, collapse | Diffuse fibrosis (fibrotic DAD), focal changes suggestive of interstitial emphysema, patchy chronic inflammation, calcifications | Recanalized thrombi |
| 18 | Basement membrane fibrosis, submucosal lymphohistiocytic and eosinophilic inflammation, intraepithelial neutrophils suggestive of superimposed infection | Organizing pneumonia, collapse, abundant hemosiderin-laden macrophages, microcystic change with bronchiolar squamous metaplasia | Diffuse fibrosis (fibrotic DAD), moderate lymphocytic/mononuclear inflammation, pulmonary interstitial emphysema | Large recanalized thrombus |
| 19 | Postmortem sloughing of epithelium, submucosal edema, mild lymphocytic inflammation | Diffuse alveolar damage, mild hemorrhage, reactive pneumocytes | Mild but diffuse interstitial lymphocytic inflammation, edema | Focal perivascular edema |
DAD, diffuse alveolar damage; PH, pulmonary hypertension.
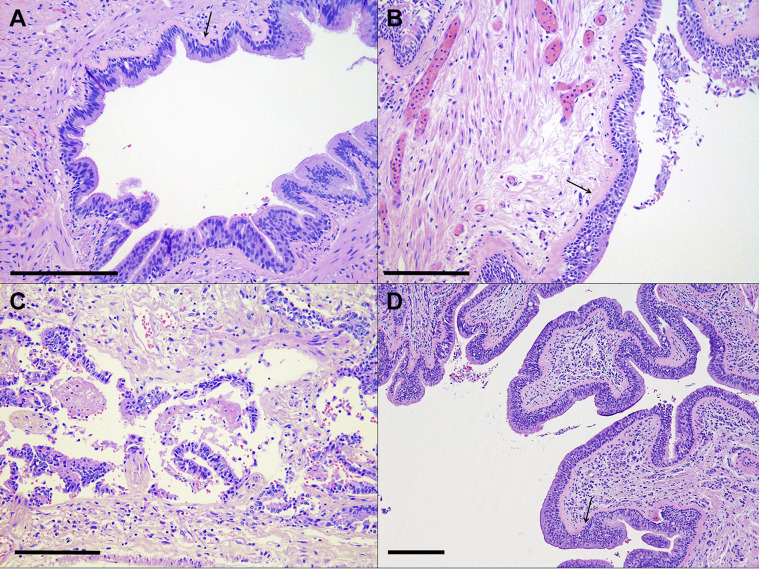
Figure 3.
Representative histologic images (hematoxylin and eosin stain; original magnification ×20) of the airways. (A) COVID-19 survivor (patient 11) with basement membrane fibrosis (arrow) and scant submucosal inflammation. (B) Negative control (patient 5) shows similar basement membrane fibrosis (arrow). (C) COVID-19 end-stage lung disease (patient 19) exhibits postmortem sloughing and scant lymphocytic inflammation in the submucosa. (D) COVID-19 end-stage lung disease (patient 17) with basement membrane fibrosis (arrow) and moderate submucosal and intraepithelial inflammation. Scale bars (bottom left corner): ∼250 μm.
Alveoli
There were high rates of emphysema in COVID-19 survivors (92%) and controls (100%) (Figures 4 A, 4B). Smoker’s macrophages, metaplastic changes, and occasional poorly formed granulomas were seen in the alveolar spaces in a subset of both groups as well (Table 3). In patient 19, alveolar COVID-19 disease was characterized by findings of DAD (Figure 4C). In patient 18, scattered intra-alveolar macrophages and rare foci of organizing pneumonia were seen within the alveoli (Table 3).
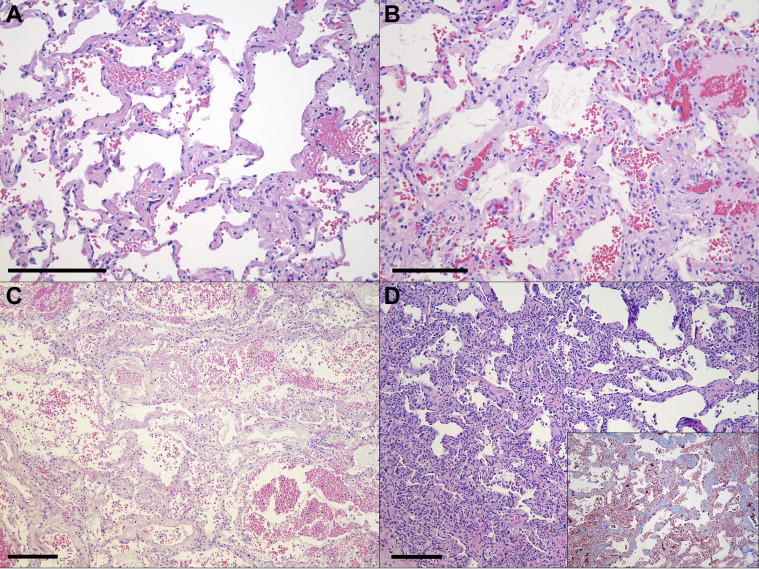
Figure 4.
Representative histologic images (hematoxylin and eosin stain) of the alveoli and interstitial regions. (A) COVID-19 survivor (patient 10) with mild, variable interstitial thickening (original magnification ×20). (B) Negative control (patient 5) shows similar interstitial thickening (original magnification ×20). (C) COVID-19 end-stage lung disease (patient 19) exhibits diffuse alveolar damage (original magnification ×10). (D) COVID-19 end-stage lung disease (patient 17) with scattered intra-alveolar macrophages and fibrotic diffuse alveolar damage (original magnification ×10), with interstitial fibrosis supported by positive trichrome staining in the interstitium (inset original magnification ×10). Scale bars (bottom left corner): ∼250 μm.
Interstitium
Areas of mild fibrosis were seen in COVID-19 survivors and controls, with 50% of COVID-19 survivors having focal fibrosis and 60% of control patients having some areas of fibrosis (Figures 4A, 4B). The pattern of fibrosis seen in COVID-19 survivors and control patients was largely attributable to smoking-related changes. The interstitium of patient 19 was inflamed with predominantly lymphocytic inflammation and edema (Figure 4C). This was in contrast to patient 17, in whom the interstitium was diffusely fibrotic (Figure 4D) with a brisk inflammatory infiltrate composed primarily of lymphocytes and was not the same pattern of fibrosis seen in COVID-19 survivors and controls.
Vasculature
Histologic evidence of mild pulmonary hypertension was present in 42% of COVID-19 survivors and in 60% of controls, but the vasculature was otherwise unremarkable (Figures 5 A, 5B). There was no significant vasculitis in any of the assessed COVID-19 survivors or the end-stage patients. While scattered microthrombi and gross pulmonary emboli have been reported in the acute COVID-19 setting,16 the vessels in patient 19 were patent, with no significant inflammation or occlusive thrombi (Figure 5C). Areas of recanalized thrombi were, however, rarely seen in patient 17 (Figure 5D).
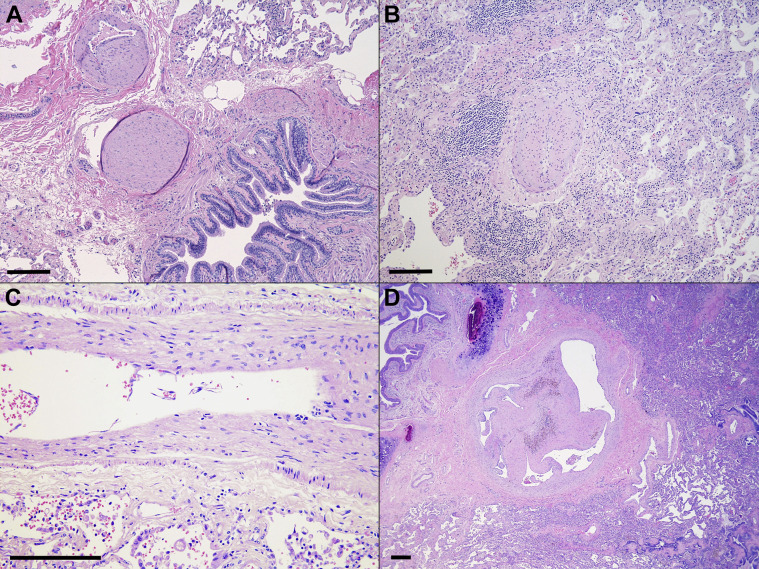
Figure 5.
Representative histologic images (hematoxylin and eosin stain) of pulmonary vasculature. (A) COVID-19 survivor (patient 10) with pulmonary hypertension (original magnification ×10). (B) Negative control (patient 4) with similar pulmonary hypertension as well as mild interstitial inflammation (original magnification ×10). (C) COVID-19 end-stage lung disease (patient 19) with minimal vascular changes (original magnification ×20). (D) COVID-19 end-stage lung disease (patient 17) shows a recanalized thrombus (original magnification ×20 magnification). Scale bars (bottom left corner): ∼250 μm.
Blinded pathologist review
An experienced pulmonary pathologist blinded to the clinical histories for each patient was unable to distinguish between COVID-19 survivors and controls in any of the assessed compartments. In addition, no significant differences were appreciated between the COVID-19 survivors who had an asymptomatic, moderate, or severe disease course in any of the assessed compartments.
Comment
In this observational study of patients who survived COVID-19, recovered, and became asymptomatic and then underwent an elective lung resection for other indications, we examined the potential for any postacute histopathologic lung changes in COVID-19 survivors. We found no discernible histopathologic changes suggesting permanent parenchymal damage in recovered COVID-19 survivors.
It is reassuring that none of the COVID-19 survivors included in this study had any lasting damage that was directly attributable to COVID-19. These findings fill an important gap in our understanding of the COVID-19 pandemic,17 especially as we move into its survivorship phase. Clinicians and patients alike now have observational evidence that once patients recover from their disease, they are unlikely to have permanent lung parenchymal sequelae at least up to 4 months from the infection, which was the maximal duration of this study.
A strength of this study is the rigorous use of a blinded pathology methodology in analyzing the difference between COVID-19 survivors’ lung tissue and non–COVID-19 infected controls, while also providing a COVID-19 end-stage lung disease comparison group. With this approach, we were able to obtain unbiased, dependable results that showed no discernible differences between the lungs of patients with COVID-19 and controls. In addition, the systematic examination across the 4 histologic compartments provided a complete picture of the airways, alveoli, interstitium, and vasculature, all of which can be affected as shown in the comparison group with COVID-19 end-stage lung disease.
On the other hand, the patients with COVID-19 end-stage lung disease likely represent those who have progressed to fibrosis as a result of organization of the original acute lung injury, a phenomenon that has been reported both before the COVID-19 pandemic18 , 19 and in severe cases of acute COVID-19.20 The reasons some patients progress to COVID-19 end-stage lung disease while others recover with pathology undiscernible from controls is unclear. We included in the present study the spectrum of COVID-19 disease courses from asymptomatic to severe disease requiring ICU admission. It is noteworthy that even in those with severe disease, their recovery was complete without notable lasting parenchymal damage.
COVID-19 can severely injure vascular endothelium and can progress to acute respiratory distress syndrome and viral pneumonia regardless of age or preexisting conditions.21 This was the case for the end-stage patients included in this study. The changes in these patients were consistent with fibrotic DAD22, 23, 24, 25 as well as diffuse lymphocytic inflammation, pulmonary interstitial emphysema, and recanalized thrombi. The cumulative changes in these 3 patients were entirely attributable to the subsequent organization of diffuse acute lung injury (ie, DAD and organizing pneumonia) and need for prolonged mechanical ventilation.
This investigation further reveals important information regarding the safety of lung resection in COVID-19 survivors. No postoperative complications occurred in the patients in this series. We found that the lung parenchyma did not exhibit clinically relevant changes that would hamper or prolong postoperative recovery after lung resection. This is valuable information to surgeons and patients when discussing potential surgical risks in this growing subset of patients. In addition, previous studies have suggested that elective operations be postponed by 6 weeks to maximize safety.26
This study has several limitations. This is an observational study subject to inherent confounding and selection bias. It is possible that the surgeons selected patients to undergo lung resection who would have a low probability of long-standing parenchymal changes. Second, most of the patients had smoking-related parenchymal changes that may obscure any possible COVID-19–related changes. However, the absence of any discernible differences and the inability of a blinded pathologist to differentiate between COVID-19 survivors and controls makes subtle changes less likely.4 , 27
In addition, we did not have data on pulmonary function tests before and after COVID-19 infection, which can meaningfully add to the overall functional assessment of these patients beyond the histopathologic assessment.
Finally, the study is limited by its sample size, although we included patients along the spectrum of COVID-19 disease course.
Notwithstanding these limitations, these results report the histopathology of COVID-19 survivors’ lungs and expand the literature surrounding the survivorship of COVID-19.
In conclusion, we did not find any distinct postacute histopathologic changes among COVID-19 survivors, who have recovered and became asymptomatic, to suggest permanent pulmonary damage. COVID-19 survivors, who are asymptomatic at time of surgical evaluation, can undergo lung resection safely without added risk, and their histopathologic findings in the otherwise benign lung parenchyma are indistinguishable from controls. This suggests that patients who have asymptomatic, moderate, or severe acute COVID-19 courses can have a full recovery. Future studies examining why some patients achieve full recovery while others go on to develop end-stage lung disease are warranted.
References
- 1.Johns Hopkins Medicine Coronavius Resource Center. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/ Accessed February 13, 2021.
- 2.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wichmann D., Sperhake J.-P., Lütgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharat A., Querrey M., Markov N.S., et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abe4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Dong C., Hu Y., et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020;296:E55–E64. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tenforde M.W., Kim S.S., Lindsell C.J., et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network — United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honigsbaum M., Krishnan L. Taking pandemic sequelae seriously: from the Russian influenza to COVID-19 long-haulers. Lancet. 2020;396:1389–1391. doi: 10.1016/S0140-6736(20)32134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carfì A., Bernabei R., Landi F. Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin R. As their numbers grow, COVID-19 “long haulers” stump experts. JAMA. 2020;324:1381–1383. doi: 10.1001/jama.2020.17709. [DOI] [PubMed] [Google Scholar]
- 10.Weerahandi H., Hochman K.A., Simon E., et al. Post-discharge health status and symptoms in patients with severe COVID-19. J Gen Intern Med. 2021;36:738–745. doi: 10.1007/s11606-020-06338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McArthur L., Sakthivel D., Ataide R., Chan F., Richards J.S., Narh C.A. Review of burden, clinical definitions, and management of COVID-19 cases. Am J Trop Med Hyg. 2020;103:625–638. doi: 10.4269/ajtmh.20-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li R., Pei S., Chen B., et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verity R., Okell L.C., Dorigatti I., et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323:2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 16.McMullen P.D., Cho J.H., Miller J.L., Husain A.N., Pytel P., Krausz T. A Descriptive and quantitative immunohistochemical study demonstrating a spectrum of platelet recruitment patterns across pulmonary infections including COVID-19. Am J Clin Pathol. 2021;155:354–363. doi: 10.1093/ajcp/aqaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yelin D., Wirtheim E., Vetter P., et al. Long-term consequences of COVID-19: research needs. Lancet Infect Dis. 2020;20:1115–1117. doi: 10.1016/S1473-3099(20)30701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kligerman S.J., Franks T.J., Galvin J.R. From the radiologic pathology archives: organization and fibrosis as a response to lung injury in diffuse alveolar damage, organizing pneumonia, and acute fibrinous and organizing pneumonia. RadioGraphics. 2013;33:1951–1975. doi: 10.1148/rg.337130057. [DOI] [PubMed] [Google Scholar]
- 19.Katzenstein A.L., Bloor C.M., Leibow A.A. Diffuse alveolar damage—the role of oxygen, shock, and related factors. A review. Am J Pathol. 1976;85:209–228. [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y., Wu J., Wang S., et al. Progression to fibrosing diffuse alveolar damage in a series of 30 minimally invasive autopsies with COVID-19 pneumonia in Wuhan, China. Histopathology. 2021;78:542–555. doi: 10.1111/his.14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson P.G., Qin L., Puah S.H. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust. 2020;213:54–56.e1. doi: 10.5694/mja2.50674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.-Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menter T., Haslbauer J.D., Nienhold R., et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.COVIDSurg Collaborative; GlobalSurg Collaborative Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia. 2021;76:748–758. doi: 10.1111/anae.15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaefer I.-M., Padera R.F., Solomon I.H., et al. In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19. Mod Pathol. 2020;33:2104–2114. doi: 10.1038/s41379-020-0595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]