Figure 11. Principles of ATP and GTP selectivity in NMP kinases.
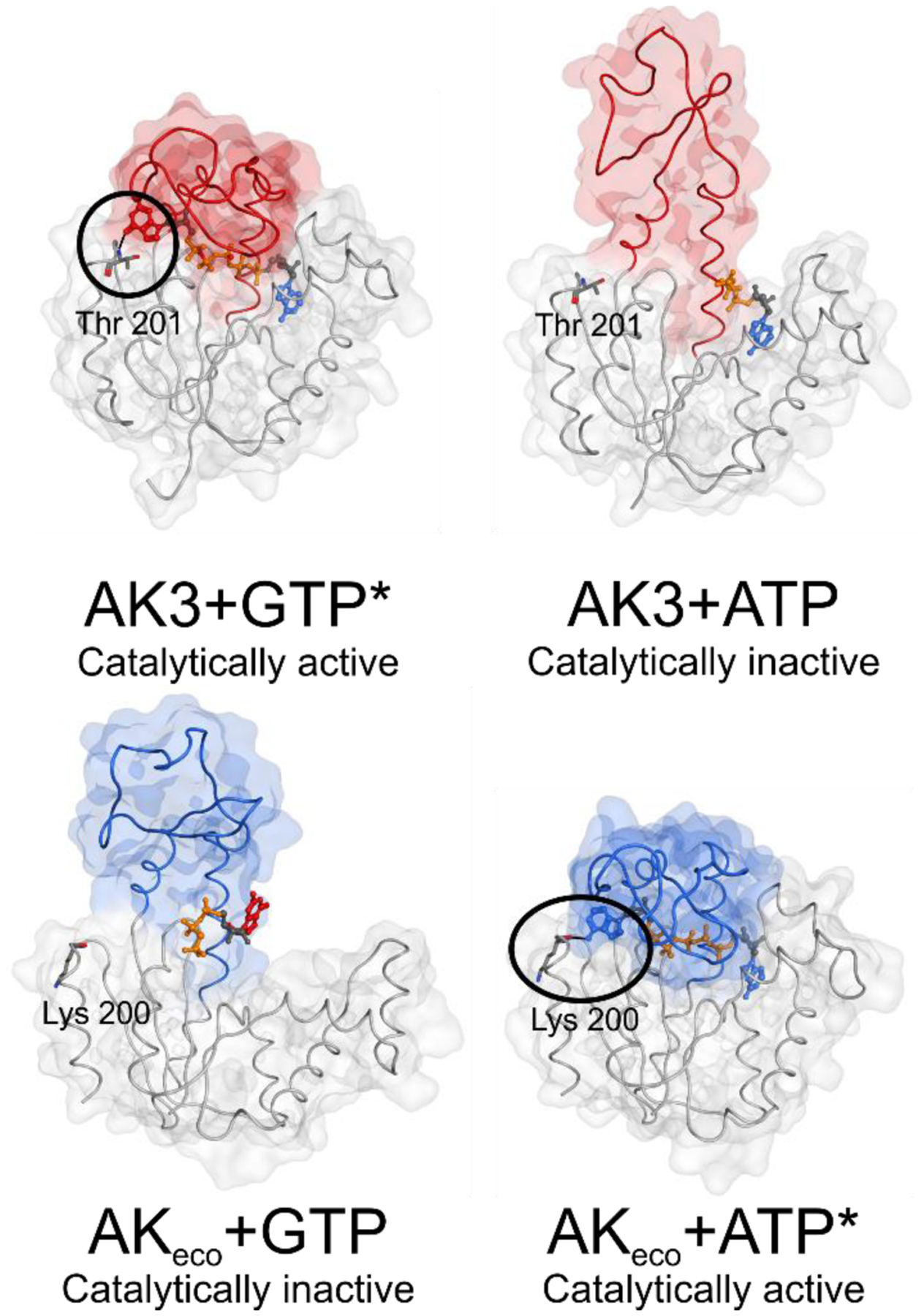
The principles are based on the findings for AK3 (GTP specific NMP kinase) presented here and for AKeco (ATP specific NMP kinase) described previously13. The function of the enzymes is dependent on positive selection via formation of a catalytically active, closed, state with the “correct” substrate, and negative selection via formation of a catalytically inactive, open, complex with the “incorrect” substrate. Top: For AK3 the catalytically active complex (left panel, PdB entry 6ZJB) with the endogenous substrate GTP is, in part, dependent on a hydrogen bond between the backbone amide proton of Thr201 and the O6 atom of the guanosine base (encircled). A catalytically incompetent state of AK3 in complex with ATP (right panel, PdB entry 6ZJD) is formed by coordination of ATP to the AMP binding domain. Bottom: In the case of the ATP specific NMP kinase AKeco a catalytically incompetent state with GTP (left panel) is obtained through arrest of an open conformation mediated through contacts with the guanosine base by residues in the ATP binding domain (PdB entry 6F7U13). The catalytically competent state of AKeco with ATP (right panel) is, in part, stabilized with a backbone hydrogen bond between Lys200 and the N6 hydrogen of the adenosine base (encircled) (PdB entry 1AKE29).
*Figures of the active complexes are shown with the inhibitors Gp5A and Ap5A for AK3 and AKeco respectively, showing the closed state of the NTP binding lid.
