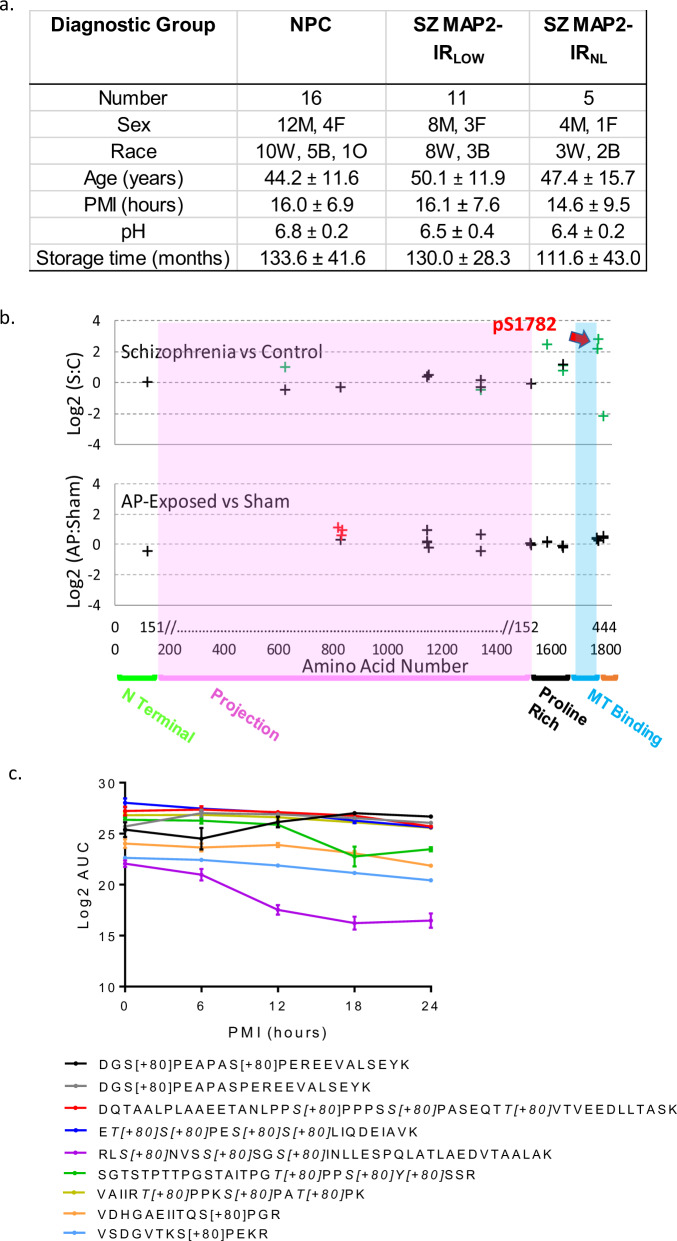
Fig. 1. MAP2 is differentially phosphorylated in Sz.
a Data for continuous variables are presented as group average ± standard deviation for each group (NPC = nonpsychiatric control, SZ MAP2-IRLOW, SZ MAP2-IRNL). There was no significant difference amongst groups on any of the variables (all p > 0.1). b Top panel shows Sz vs NPC, phosphopeptides are denoted by crosses at the position of their starting amino acid. Nine phosphopeptides had significant alterations (FDR corrected q < 0.05, green crosses). Note significantly altered phosphopeptides were present in the Projection domain (unique to high MW MAP2A/B) and surrounding the MT-binding domain. Red arrow denotes pS1782. No phosphopeptides were detected in the MT-binding domain. Bottom panel shows antipsychotic-exposed (AP) vs sham-exposed monkey. Three peptides were nominally significant (p < 0.05, red crosses). Most peptides were identified in both species (indicated by alignment on x-axis), but peptides significantly altered in Sz did not correspond to those increased by antipsychotic exposure. x-axis shows amino acid location in MAP2C and in canonical MAP2B. MAP2 functional domains are denoted. c Log2 area under the curve (AUC) values for significant MAP2 phosphopeptides across increasing postmortem intervals in mouse tissue demonstrates relatively linear stability that is easily accounted for in our PMI matching of subjects. The one exception to these linear trends is RLS[+80]NVSS[+80]SGS[+80]INLLESPQLATLAEDVTAALAK. Individual peptides indicating phosphorylated residue(s) are listed in the legend below the graph. Data shown are ±SEM from N = 4 samples per timepoint.