Fig. 3. Computational modeling of MAP2 pS1782 alters predicted structure and reduces microtubule binding.
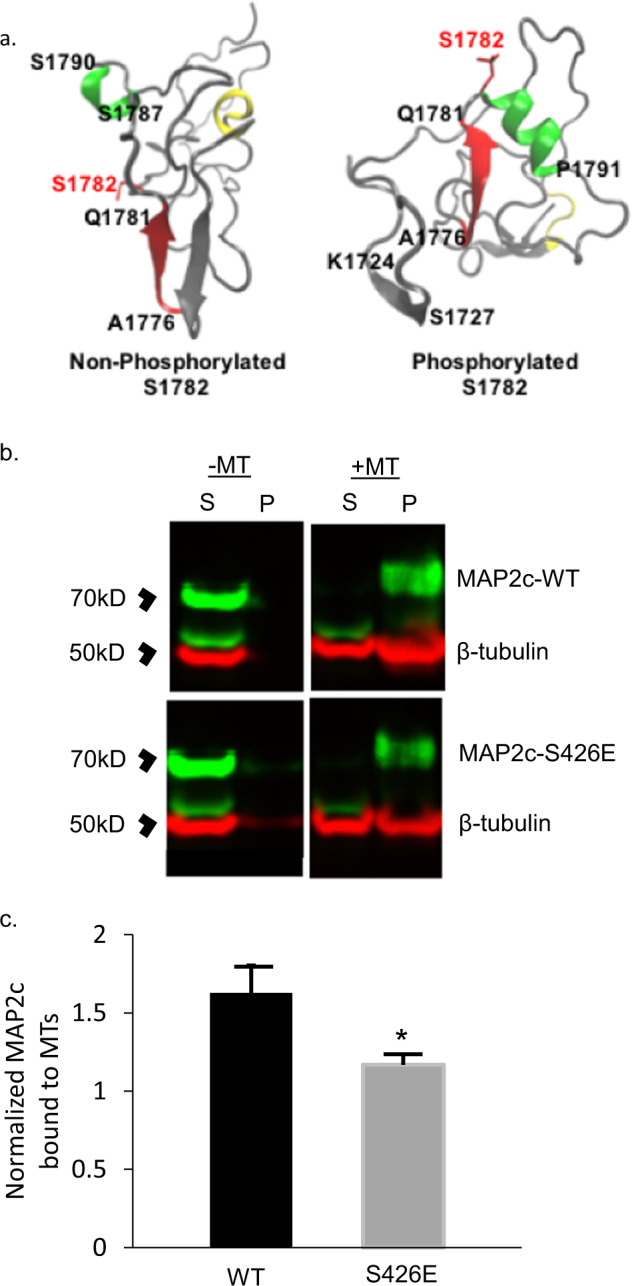
a Snapshot of the C-terminal domain of MAP2. Amino acids A1776 to Q1781 form an extended β strand (red β strand) that is occluded by an opposing β strand. In much of the nonphosphorylated ensemble, there is found to be helical propensity in or near regions S1759 to R1765 (yellow) which is not found in the phosphorylated ensembles. In pMAP2 (pS1782), the β strand at A1776 to Q1781 is no longer occluded by another β strand; this is predicted to reduce microtubule binding and enhance interactions with other proteins. The effect of phosphorylation is recapitulated by phosphomimetic mutation S1782E (data not shown). b Reduced MT binding in MAP2c with the phosphomimetic mutation S426E, homologous to S1782 in full-length MAP2b relative to MAP2c-WT. 30 µL of cell lysate from transiently transfected HEK293 cells was subjected to in vitro MT-binding assay and supernatant (S) and pellet (P) fractions were subjected to SDS-PAGE. c Densitometric analysis of gels shows decreased MT binding in the S426E mutant compared to WT. Data shown are from three independent experiments, ±SEM. *p < 0.05.
