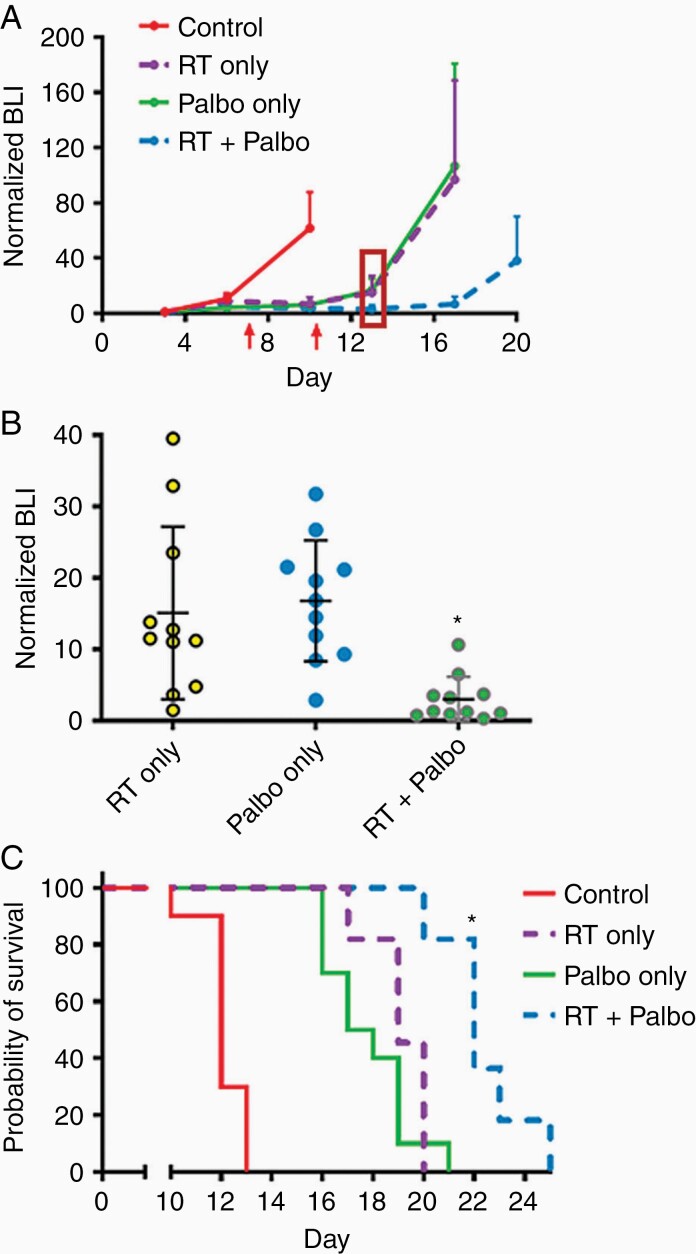
Figure 3.
In vivo activity of palbociclib and hypofractionated RT against CH157 meningiomas. BLI monitoring (A & B) and survival analysis (C) of mice with intracranial CH157 tumor, and treated with RT only, palbociclib only, or RT + palbociclib. RT administered days 3–7 post tumor cell injection: 2 Gy/day × 5 days. Palbociclib administered days 3–10 post tumor cell injection 150 mg/kg/day. The first red arrow in (A) indicates the last day of RT. The second red arrow indicates the last day of Palbociclib. In (B), *P < 0.01 for tumor bioluminescence of RT + palbociclib mice vs. RT only or palbociclib only mice in (B), as determined at 3 days following completion of palbociclib administration (indicated by the red rectangle in (A)). For (C), *P < 0.05 for all two-way survival comparisons, except for RT only vs. RT+palbociclib (P = 0.17). N in (C) = 10 mice per group for control and palbociclib only, N = 11 for RT and RT + palbociclib.