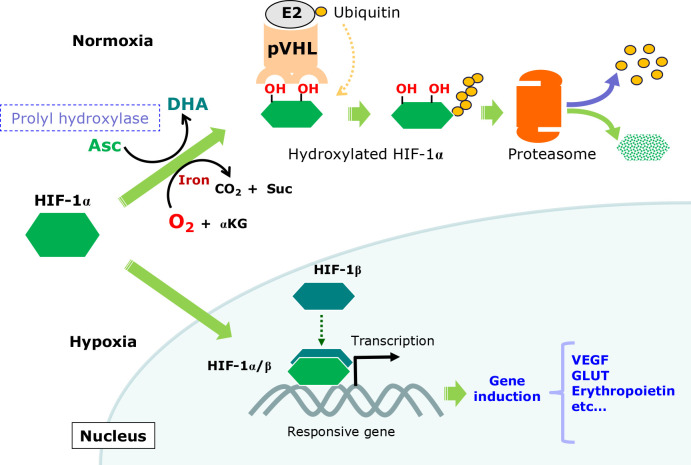
Fig. 7.
Roles of Asc in controlling HIF-1α action. Under normoxic conditions, prolyl hydroxylase catalyzes the hydroxylation of specific proline residues in HIF-1α, which leads to polyubiquitination by the ubiquitin ligase activity of pVHL, followed by degradation via proteasomes. Under hypoxic conditions, the short oxygen supply suppresses proly hydroxylation, which results in the stabilization of HIF-1α. After translocation to the nucleus, HIF-1α dimerizes with HIF-1β, which is constitutively present largely in the nucleus, and together with other transcriptional factors, activates transcription of corresponding genes, such as erythropoietin, vascular epidermal growth factor (VEGF), and GLUT.