Fig. 6.
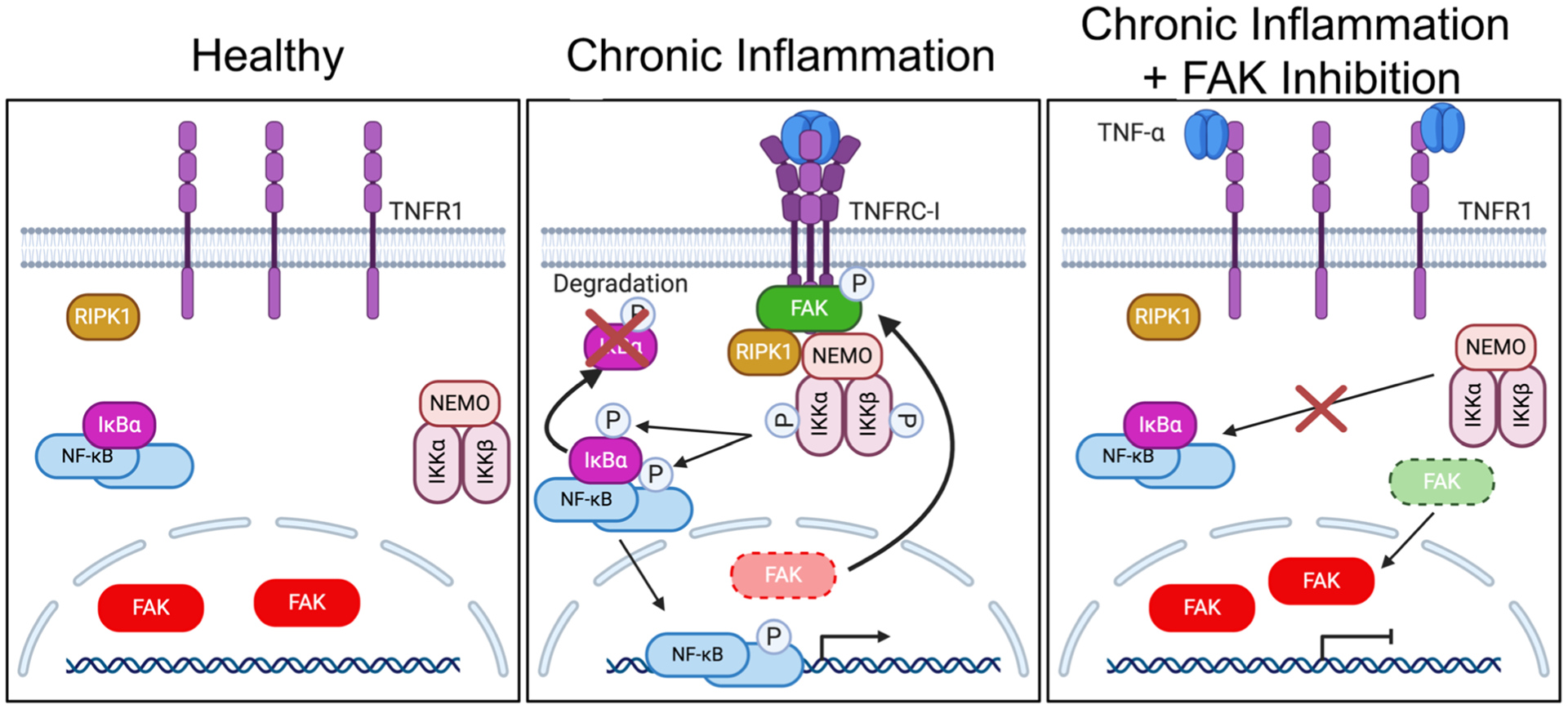
FAK cytoplasmic localization and activity are important for TNFRC-I formation and IKK-NF-κB activation. Left: Under healthy conditions, FAK is mainly localized to the nucleus of ECs. This increased nuclear FAK is associated with inactive NF-κB sequestered by IκBα proteins in the cytoplasm. Middle: Under chronic inflammation, such as continued TNF-α stimulation, trimerization of TNFR1 leads to FAK activation and redistribution of FAK from the nucleus to the cytoplasm. Active FAK increases recruitment of RIPK1 and the IKK complex to TNFRC-I. After activation, the IKK complex promotes IκBα degradation and activates NF-κB, which enters the nucleus and promotes inflammatory gene transcription. Right: FAK inhibition decreases recruitment of RIPK1 and the IKK complex to TNFR1, thus blocking formation of TNFRC-1 and decreasing sustained activation of the IKK complex and NF-κB.
