Fig.4. Changes in Injection-site Swelling, Reddening, and Pain in the TIV+PPSV23 and TIV+Placebo Groups (Left Upper Arm).
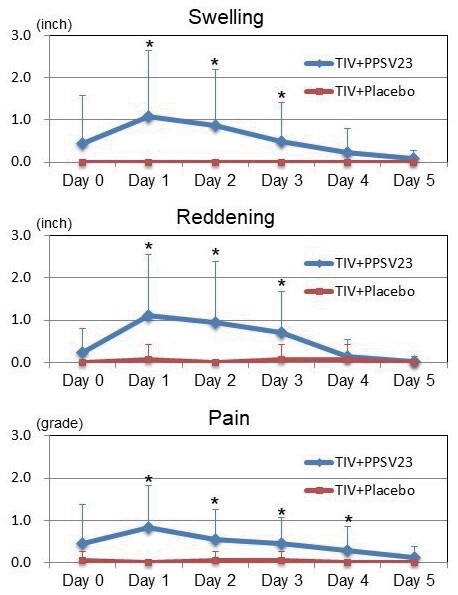
In the left upper arm, the TIV+PPSV23 group had significantly more swelling at Day 1 (1.1±1.6 vs. 0.0±0.0 inches; P <0.01), Day 2 (0.9±1.3 vs. 0.0±0.0 inch; P <0.01), and Day 3 (0.5±0.9 vs. 0.0±0.0 inch; P <0.01), significantly more reddening at Day 1 (1.1±1.5 vs. 0.1±0.3 inches; P <0.01), Day 2 (0.9±1.4 vs. 0.0±0.0 inches; P <0.01), and Day 3 (0.7±1.0 vs. 0.1±0.3 inches; P <0.05), and significantly higher pain grading at Day 1 (0.8±1.0 vs. 0.0±0.0; P <0.01), Day 2 (0.6±0.7 vs. 0.0±0.2; P <0.01), Day 3 (0.4±0.6 vs. 0.0±0.2; P <0.01), and Day 4 (0.3±0.6 vs. 0.0±0.0; P <0.05) compared to the TIV+Placebo group.
Each error bar (1SD) was demonstrated by one-side because of retativly large values of SD. TIV; trivalent influenza vaccine, PPSV23; 23-valent pneumococcal polysaccharide vaccine.
* P <0.05 compared with the TIV+Placebo group.
