Summary
The focus of this review is to examine the role of ITK signaling in multiple diseases and investigate the clinical potential of ITK inhibition. The diseases and potential interventions reviewed include T cell-derived malignancies as well as other neoplastic diseases, allergic diseases such as asthma and atopic dermatitis, certain infectious diseases, several autoimmune disorders such as rheumatoid arthritis and psoriasis, and finally the use of ITK inhibition in both solid organ and bone marrow transplantation recipients.
Subject areas: Biological sciences, Biochemistry, Molecular biology, Immunology, Cell biology
Graphical abstract
Biological sciences; Biochemistry; Molecular biology; Immunology; Cell biology
Introduction
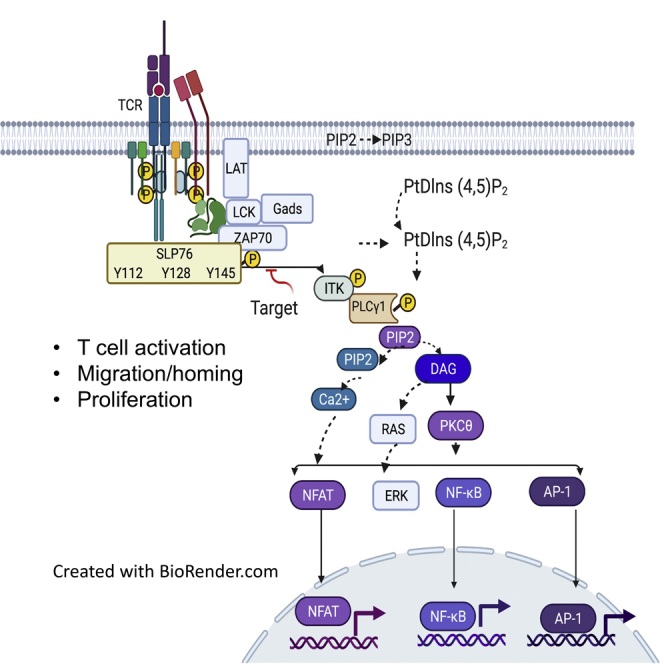
IL-2-inducible T cell kinase (ITK) is essential for proximal T cell receptor (TCR) signaling. Following TCR ligation, ITK is recruited to the cell membrane where it interacts with Linker for activation of T cells (LAT) and SH2-domain-containing leukocyte protein of 76 kDa (SLP76) (Qi et al., 2006; Bunnell et al., 2000). Proper positioning of ITK and Phospholipase Cγ1 (PLCγ1), the enzyme and the substrate, respectively, within the LAT-SLP76 complex leads to the phosphorylation and activation of PLCγ1 (Liu et al., 1998; Berg et al., 2005). Investigation of ITK signaling in disease pathogenesis and the potential efficacy of its inhibition in malignant, autoimmune, and other illnesses is an exciting area of both pre-clinical and clinical study (Lechner et al., 2020). Ibrutinib is a potent inhibitor of BTK and is already US Food and Drug Administration (FDA) approved to treat B cell leukemia and lymphomas. However, owing to the structural homology of ITK and BTK, ibrutinib has been shown to be efficacious against lymphomas in preclinical and clinical studies through its action on ITK signaling (Sagiv-Barfi et al., 2015; Dubovsky et al., 2013). ITK and BTK are members of the Tec family of kinases, which are primarily expressed in cells of hematopoietic lineage (Felices et al., 2007). Tec family kinase structure includes a C terminus kinase domain, Src homology (SH)2 domain, SH3 domain, and a proline-rich region located within the N terminus.
The tyrosine residue in the 145 position (Y145) of SLP76 is particularly crucial for ITK activation and signal propagation (Jordan et al., 2006; Fang et al., 1996). In our laboratory, we are studying a peptide that specifically targets and prevents the phosphorylation of SLP76 at the Y145 position (Mammadli et al., 2021a). We believe that the investigation of SLP76 as a novel drug target will lead to the development of more desirable and targeted treatments by decreasing the off-target inhibition of various tyrosine kinases (Figure 1). In this review, we highlight how ITK signaling can lead to aberrant immune behavior and disease pathogenesis in various malignant and immunological disorders. We briefly review the clinically relevant material, examine the efficacy of ITK inhibition in disease models and clinical studies, postulate potential avenues for further research, and, finally, discuss the results of our novel SLP76 inhibitor in a murine model of graft-versus-host disease.
Figure 1.
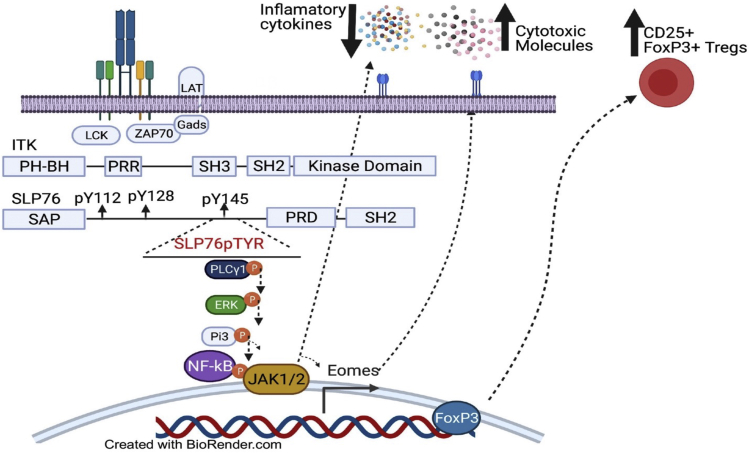
Targeting SLP76:ITK interaction a with a novel and specific inhibitors has a therapeutic potential
To generate a molecule that specifically inhibits the interaction between pY145 of SLP76 and the SH2 domain of ITK, we designed a peptide based on the amino acid sequence of SLP76 from N132 to A155, which contains a phosphorylated tyrosine residue at Y145. The interaction between ITK and SLP76 involves the phospho-tyrosines at position pY145 and the proline-rich domain (PRD) of SLP76 docking onto the SH2 and SH3 domains of ITK, respectively. This multivalent anchoring of ITK on the different SLP76 docking sites results in distinct downstream signaling effects. This SLP76pTYR peptide significantly reduced IFN-γ and TNF-α production by TCR-stimulated T cells. Disrupting the SLP76:ITK interaction also significantly reduces PLC-γ1, ERK, P13K, and NF-κB phosphorylation. Inhibition of ITK enhances FOXP3+ regulatory T cells.
T cell lymphoma and a novel immunotherapeutic approach
Peripheral T cell lymphoma
Peripheral T cell lymphoma (PTCL) accounts for ∼10%–15% of all non-Hodgkin's lymphomas (Sabattini et al., 2010). Compared with their B cell counterparts, T cell malignancies are rarer, more difficult to accurately diagnose, have an increased predisposition to develop resistance to treatments, and are therefore more deadly (Vose et al., 2008; Kim et al., 2003). PTCL is highly heterogeneous, both clinically and histologically, with the most common subtypes including angioimmunoblastic T cell lymphoma, anaplastic lymphoma kinase-negative anaplastic large cell lymphoma, and peripheral T cell lymphoma, not otherwise specified (Sabattini et al., 2010). Despite this heterogeneity, the majority of PTCL cases are treated with the indiscriminate chemotherapy regimen CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) (Moskowitz et al., 2014), which is commonly used for B cell-derived malignancies. The development of targeted treatments for PTCL would revolutionize care, the same way targeted therapies for B cell malignancies have revolutionized care for the past two decades.
Multiple investigations have elucidated that the formation of an ITK-SYK fusion protein may be essential for T cell oncogenesis (Moskowitz et al., 2014; Pechloff et al., 2010). These investigations show that ITK-SYK fusion protein tend to associate with lipid rafts and are capable of phosphorylating and activating TCR proximal intracellular signaling proteins (like SLP-76) in an antigen-independent manner. This interaction results in an increase in CD69 expression and IL-2 production. The prevalence of the ITK-SYK gene fusion may be as high as 17% in patients with the PTCL, not otherwise specified subtype (Streubel et al., 2006), although more robust studies are necessary to establish a more accurate prevalence. Another novel ITK fusion protein, ITK-FER, was recently discovered in PTCL patient samples and shown to promote colony formation (Boddicker et al., 2016). Although ITK fusion proteins have not been found in other PTCL subtypes, studies have shown an increase in ITK expression in the other subtypes (Agostinelli et al., 2014; Liang et al., 2014). Intracellular signaling through ITK may also play an integral role in the development of chemotherapy resistance in T cell populations (Wang et al., 2017b), further highlighting the importance of developing novel ITK inhibitors followed by rigorous clinical investigation.
More recently, pre-clinical studies have shown that ITK inhibitors were successful in suppressing tumor growth in models of T cell lymphoma (Li et al., 2020). A topical retinoid was shown to inhibit ITK and reduce tumor growth in a murine model of cutaneous T cell lymphoma (Li et al., 2020). Another study showed that ITK inhibition worked synergistically with chemotherapy in a T cell lymphoma xenograft model (Liu et al., 2019), and ibrutinib was shown to shrink tumor burden using a novel T cell lymphoma murine model (Allchin et al., 2019). However, despite these findings, a recent pilot study found that 14 patients with refractory T cell lymphoma failed to respond to ibrutinib (Kumar et al., 2018).
A novel immunotherapeutic approach
Malignancies, autoimmune diseases, and even parasitic and other infectious diseases are often a consequence of, or result in, aberrant T cell fates. A common form of immune subversion by malignancies is skewing of helper T cell populations toward Th2 cells within the tumor microenvironment. This results in a reduced capacity of the adaptive immune system to recognize and kill tumor cells. It is well established that ITK signaling plays an integral role in the generation of Th2 responses and is therefore essential for the humoral responses and B cell functions orchestrated by the adaptive immune system (Andreotti et al., 2010). ITK signaling is also essential for normal T cell development (Liao and Littman, 1995). Multiple studies have shown that ITK-knockout and/or ITK-mutant mice are unable to launch Th2 responses despite exposure to conditions that typically elicit strong humoral activity (Fowell et al., 1999; Schaeffer et al., 2001). Not only do knockout or mutant ITK conditions result in blunted Th2 function, but they also additionally result in increased expression of T-bet and Th1-related cytokines, under the same conditions that typically elicit a Th2 response (Miller et al., 2004). More recent evidence has shown that IL-4, often associated with parasitic infections and subsequent Th2 responses, as well as allergic diseases, causes expansion of Th1 cells under ITK-knockout conditions (Kannan et al., 2017). ITK-knockout or ITK-dysfunctional T cells are not anergic and impaired; instead, they are transformed into powerful elicitors of cytotoxic activity with the potential to fight cancer. The Th1 lineage of CD4 cells is responsible for cellular immunity and therefore is essential for the immune system's anti-tumor capabilities. Pre-clinical and clinical studies have shown promise in increasing the Th1/Th2 ratio to bolster the anti-tumor response as an effective treatment of malignancies (Andreotti et al., 2010; Miller et al., 2004). One study showed that intratumor injections of IL-12 mRNA resulted in significant tumor regression, depending on a Th1 transformation of the tumor microenvironment, increases in IFN-γ production, and subsequent CD8 function (Hewitt et al., 2020). A separate study showed that a compound (FBrf0243740) isolated from a traditional Chinese herb could also induce a Th1 transformation and decrease tumor burden in breast cancer models nearly as well as the commonly used estrogen receptor blocker, tamoxifen (Zhao et al., 2019).
Other more clinically relevant studies have shown that ibrutinib can induce a Th1 transformation; it may be an effective treatment of hematological malignancies, solid tumors, and infectious diseases; and it may even help with the in vitro expansion of CAR-T cells (Schaeffer et al., 2001; Kannan et al., 2017). Ibrutinib was also shown to be effective in treating visceral leishmaniasis in a preclinical model (Varikuti et al., 2019). Serum cytokine analysis of patients with chronic lymphocytic leukemia (CLL) receiving ibrutinib showed an increase in the Th1-related cytokine IFN-γ and a decrease in the Th2-related cytokines IL-10, IL-4, and IL-13 (Dubovsky et al., 2013). Ibrutinib decreased the expression of the immune checkpoint molecules PD-1 and CTLA-4 by CLL cells in vivo (Long et al., 2017). Ibrutinib was also shown to be effective in improving the yield and development of CAR-T cells in CLL patient samples, which has remained difficult compared with other B cell malignancies treated with CAR-T cells (Fan et al., 2021). All of these studies highlight the potential of ITK inhibition as a novel immunotherapeutic approach to cancer treatment.
Allergic diseases
Asthma
Aberrant Th2 function is observed in and may be the primary driver of many allergic disorders. As previously stated, ITK signaling is essential for Th2 differentiation and subsequent production of related cytokines (Andreotti et al., 2010). Asthma is one of the most common chronic diseases with a worldwide prevalence of 4.3% in adults (Papi et al., 2018). Asthma is a heterogeneous condition most often mediated by Th2 function and production of IL-5, IL-4, IL-9, and IL-13. These cytokines initiate IgE class switching, mast cell activation, and the recruitment of eosinophils and other granulocytes. This ultimately leads to airway hyperresponsiveness, smooth muscle hypercontractility, and airway remodeling. Targeted therapies for asthma, such as monoclonal antibodies against IgE and IL-5, have proven to be effective in patients who fail to improve with standard therapy (Long et al., 2017).
Multiple murine models have demonstrated the importance of ITK in asthma pathogenesis (Mueller and August, 2003; Ferrara et al., 2006; Gomez-Rodriguez et al., 2016). These studies showed significantly reduced cytokine-mediated lung inflammation and mast cell degranulation, as well as decreased airway hyperresponsiveness, in ITK-knockout or ITK-mutant mice with ovalbumin-induced asthma. Genetic studies have also revealed a polymorphism in the promoter region of ITK that increases the risk of allergic asthma (Lee et al., 2011). This suggests that ITK may be a therapeutic target for asthma; however, paradoxical results have been observed in studies investigating the efficacy of a small molecule ITK inhibitor in a murine asthma model (Sun et al., 2015; Lin et al., 2004). Surprisingly, treatment with the small molecule ITK inhibitor failed to show reduced lung inflammation and instead showed an increase in Th2-related cytokines (Sun et al., 2015). However, ibrutinib was recently shown to reduce airway inflammation in a murine model of mixed granulocytic asthma (eosinophilic and neutrophilic), a phenotypic subtype of allergic asthma that is commonly resistant to corticosteroid treatment (Nadeem et al., 2019). However, it is difficult to conclude that these observed effects are solely a result of T cell inhibition, due to the expression of BTK and other Tec family kinases on mast cells as well (Felices et al., 2007). Despite these contradictory results, further investigation into ITK as a target for asthma treatment is warranted.
Atopic dermatitis
Cutaneous disorders not only cause significant pain and discomfort but can also result in severe emotional and social stress because of their cosmetic effects. Atopic dermatitis is the most common inflammatory skin condition with a prevalence in the United States of 11.3%–12.7% and 6.9%–7.6% in children and adults, respectively (Kim et al., 2019). Patients with atopic dermatitis tend to have a history of other allergic disease (i.e., asthma, eczema) and present with pruritus and dry skin often triggered by an allergen (Jameson et al., 2018). The pathogenesis of atopic dermatitis is complex and not fully elucidated; however, skin barrier dysfunction and an aberrant upregulation of Th2 cytokines following allergen exposure are central to its development (Kim et al., 2019). Treatment of atopic dermatitis typically includes application of topical glucocorticoids, and occasionally calcineurin inhibitors. Various systemic targeted monoclonal antibodies against different interleukins have recently been FDA approved for patients who fail initial therapy.
Genetic studies have revealed that certain ITK polymorphisms are associated with an increased risk of atopy and seasonal allergies (Graves et al., 2005; Benson et al., 2009). A clinical study showed that T cells from patients with mild to severe atopic dermatitis had increased expression of ITK (Matsumoto et al., 2002). A preclinical study showed that the inhibition of ITK with a small molecule effectively reduced skin inflammation in a hypersensitivity murine model (von Bonin et al., 2011). These studies suggest that ITK may be a useful target in the treatment of atopic dermatitis. Studies examining the efficacy of a topical ITK treatment would be beneficial, since the current topical treatments for atopic dermatitis (glucocorticoids and calcineurin inhibitors) do have significant side effects, especially following prolonged use (Jameson et al., 2018).
Infectious diseases
Leishmaniasis
Leishmaniasis is caused by the eukaryotic intracellular protozoan of the genus Leishmania and is most frequently observed in developing tropical regions (Jameson et al., 2018). Most exposed individuals mount a successful Th1 immune response and clear the pathogen, but for unknown reasons, some individuals will mount a predominantly Th2-mediated response. These individuals are unable to clear the protozoa and develop severe disease of the reticuloendothelial system. The Th2-related cytokine IL-10 plays an important role in the pathogenesis, particularly due to its inhibitory role of macrophage function (Dayakar et al., 2019).
As previously discussed, ITK signaling is important for Th2 function, potentially due to regulation of GATA-3 (Andreotti et al., 2010). One murine model of cutaneous leishmaniasis showed that the use of ibrutinib was efficacious and that disease response negatively correlated with Th2 cytokine levels (Dubovsky et al., 2013). Another promising study showed that the use of ibrutinib was superior to the commonly utilized pentavalent antimonial treatment in a murine model of visceral leishmaniasis (Varikuti et al., 2019). The same study also showed an increase in IFN-γ and enhanced mature granuloma formation, leading to improved clearance of the parasite from liver tissue in ibrutinib-treated mice.
Opportunistic infections
Patients with leukemia are chronically immunosuppressed owing to the natural pathogenesis of the disease and owing to myelosuppression from chemotherapeutic regimens (Morrison, 2010). This leaves them vulnerable to opportunistic infections, which are a common cause of death in this patient population. Many patients with leukemia are on chronic antibiotic prophylaxis for opportunistic infections such as pneumocystis pneumonia (PCP) (Truong and Ashurst, 2021).
A recent study found that pre-treatment with ibrutinib improved immune responses against and clearance of Listeria monocytogenes in a murine model of leukemia (Dubovsky et al., 2013). The leukemic mice treated with ibrutinib mounted stronger and more robust immune responses compared with the untreated mice. In fact, the immune response mounted in the treated arm of the study was greater than the immune response observed in non-leukemic mice. The treated leukemic mice also cleared the infection at a similar rate as healthy mice. This study highlights the potential for ITK inhibition as an adjuvant prophylactic therapy in immunosuppressed patients.
Human immunodeficiency virus infection
From 2000 to 2016, the annual number of HIV cases fell by more than 40%, reflecting the great advances made in HIV prevention and antiretroviral therapy (Jameson et al., 2018). Despite these advances, up to one million people die every year from AIDS-related complications, and resistance to therapy is of constant concern and study. Several studies have shown that ITK plays an important role in both HIV entry and subsequent replication, effects that are largely due to ITK's regulatory function on actin polymerization (Hain et al., 2018). These studies have shown that both pharmacological inhibition of and RNA interference with ITK expression decrease viral binding and entry in either Jurket T cells or primary CD4 T cells (Hain et al., 2018; Readinger et al., 2008). One of these studies also showed that ITK signaling may be necessary for viral replication following viral entry but is not needed for reverse transcription or DNA integration (Readinger et al., 2008). Additional studies have elucidated the importance of the interaction between ITK and Nef, a transmembrane accessory protein encoded by HIV, which is highly correlated with infectivity (Tarafdar et al., 2014; Basmaciogullari and Pizzato, 2014). These studies show that Nef constitutively activates ITK in a TCR-independent manner. Of interest, ITK inhibition with a small molecule was successful at inhibiting infectivity and replication of Nef-sufficient HIV but had no effect on Nef-defective HIV, suggesting that ITK's impact on HIV functions in a Nef-dependent manner (Tarafdar et al., 2014).
Autoimmune disorders
The improper balance between proinflammatory Th17 cells and anti-inflammatory regulatory T cells (Tregs) plays an essential role in many autoimmune conditions, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple sclerosis (MS), inflammatory bowel disease (IBD), and psoriasis (Lee, 2018; Fasching et al., 2017). Differentiation of Th17 cells and Treg cells uses a common pathway mediated by TGF-β. Differentiation into either Th17 or Treg cells is regulated by additional co-stimulatory molecules present within the microenvironment of CD4 cells sensitized to TGF-β. Some examples of these co-stimulators are IL-6, which leads to Th17 differentiation, and IL-2, which causes Treg differentiation. It is well established in the literature that ITK signaling negatively regulates Treg cells and positively regulates Th17 cells (Elmore et al., 2020; Mamontov et al., 2019; Gomez-Rodriguez et al., 2009). Attenuation of TCR signaling by ITK or SLP-76 dysfunction causes upregulation of Foxp3, the master regulator of Treg differentiation. The mechanism by which ITK positively upregulates Th17 differentiation is not as well understood; however, one study showed that CD4 T cells with dysfunctional ITK differentiated into Treg cells despite stimulation with IL-6 and TGF-β (Gomez-Rodriguez et al., 2014). Further supporting ITK's role in Th17 function is a study of a patient suffering from a rare ITK deficiency, who was found to produce less Th17-related cytokines (Eken et al., 2019). These studies indicate that ITK inhibition may help to restore the aberrant Th17/Treg balance found in autoimmunity. In fact, some studies have already highlighted the potential for ITK inhibition to regulate Th17 and Treg function (Gomez-Rodriguez et al., 2014; Nadeem et al., 2020b). Recent whole genome sequencing of families with significant clustering of autoimmune disease has identified variants in the proximal TCR signaling pathway, including the formation of the LAT-SLP76 complex (Wang et al., 2020), further supporting the potential of ITK inhibition in autoimmune conditions.
Rheumatoid arthritis
RA is the most common cause of chronic inflammatory arthritis and is characterized by polyarticular joint inflammation, as well as other systemic manifestations such as rash and weight loss (Jameson et al., 2018). This disease commonly effects adult females and tends to progress in intensity. The treatment of rheumatoid arthritis has become exceedingly complex with the development of various biologic and small molecule inhibitors, but many patients continue to be treated with the powerful chemotherapeutic, methotrexate.
The classic manifestations of RA are caused by the immunological destruction of synovial tissue and underlying bone and cartilage (Jameson et al., 2018). Like many autoimmune conditions, the exact mechanism of disease pathogenesis remains elusive. However, the essential role of Th17 cells in RA pathogenesis was described in one study, which showed an increase in IL-17 expressed by Th17 cells in the synovial fluid of patients with early RA (Raza et al., 2005). Additional studies have correlated Th17 cells and Th17-related cytokine levels in the synovial fluid and serum with disease severity (Zizzo et al., 2011; Rasmussen et al., 2010). A recent phase III clinical trial of secukinumab, an IL-17A inhibitor, was shown to be efficacious in patients with RA who had failed previous therapy (Blanco et al., 2017).
As previously stated, ITK signaling positively regulates the proinflammatory effects of Th17 cells (Mamontov et al., 2019; Gomez-Rodriguez et al., 2009). A growing body of evidence suggests that BTK inhibitors are efficacious in the treatment of RA. This includes evidence that a BTK inhibitor prevents disease progression in murine models of both RA and SLE (Haselmayer et al., 2019; Cohen et al., 2020). A phase II trial of Fenebrutinib, a BTK inhibitor, also showed that modulation of BTK is efficacious in patients with RA (Cohen et al., 2020). To our knowledge, neither of these pharmacologic agents were evaluated for potential activity against ITK. Therefore, it is possible that some of the observed benefits in these studies could be due to Th17 suppression secondary to ITK inhibition.
Systemic lupus erythematosus
SLE is characterized by tissue damage mediated by autoantibodies and immune complex formation, with the potential to damage nearly every organ system (Jameson et al., 2018). Nephritis is typically the most serious manifestation, responsible for a large percentage of SLE-related mortality. Depending on the severity of the disease, SLE is treated with nonsteroidal anti-inflammatory drugs, antimalarial medications like hydroxychloroquine, and/or glucocorticoids. The use of biologics and more targeted therapies for SLE is an area of intense study, especially for the treatment of lupus nephritis.
SLE pathogenesis is initiated by the overproduction of immunogenic forms of nucleic acid as well as other self-antigens (Jameson et al., 2018). This immunogenic and inflammatory environment causes a shift in the CD4 population away from Tregs and toward Th17 and Th1 cells. Clinical studies of patients with SLE and animal models of SLE have observed an increase in Th17 function and a decrease in Treg function in both serum and tissue samples (Gomez-Rodriguez et al., 2009; Nadeem et al., 2020b). In fact, several interventions aimed at restoring Th17/Treg balance in patients with SLE have proven successful, including infusions of mesenchymal stem cells (Wang et al., 2017a), autologous Treg transplantation (Dall'Era et al., 2019), and mTOR blockade (Kato and Perl, 2018). To our knowledge, no clinical or preclinical studies have investigated the efficacy of ITK inhibition in SLE. However, recent evidence has shown an increase in expression of ITK in SLE patient samples (Xu et al., 2016), further supporting future investigation into ITK inhibitors for SLE therapy.
Multiple sclerosis
MS is an autoimmune disease of the central nervous system characterized by chronic inflammation and demyelination (Jameson et al., 2018). Onset of MS can be abrupt or insidious, with many patients experiencing visual disturbances, paresthesias, and weakness. MS is a progressive and debilitating disease, often leaving patients functionally disabled. Management of MS typically includes the use of glucocorticoids for acute attacks and long-term use of immuno-modulating agents like IFN-β and natalizumab. The treatment of MS has an additional layer of complexity compared with the other diseases covered in this review, since any potential new therapy must be able to cross the blood-brain barrier.
MS pathogenesis is rooted in the recognition of myelin basic protein by auto-reactive T cells, which leads to aberrant upregulation of Th1- and Th17-related cytokines (Jameson et al., 2018). Multiple animal models and MS patient sample studies have suggested that the Th17-related cytokines IL-17 and IL-23 may play an essential role in disease pathogenesis (Wen et al., 2012). In addition, a small clinical trial of Secukinumab, a monoclonal antibody against IL-17A, showed that inhibition of IL-17A was effective in reducing MS plaques on MRI (Havrdová et al., 2016). However, larger studies with more clinically relevant outcomes are needed. Despite the links between ITK signaling and Th17 function, there has been little investigation into ITK blockade and its effects on MS pathogenesis. A single study did reveal that ITK-knockout mice, as well as mice who received an autologous graft of ITK-knockout CD4 T cells, had less severe disease in a murine model of MS (Kannan et al., 2015).
Inflammatory bowel disease
IBD is an immune-mediated chronic condition with two subtypes: ulcerative colitis and Crohn's disease (Jameson et al., 2018). Depending on the subtype, patients typically present with abdominal pain, diarrhea, and rectal bleeding. Treatment often includes 5-ASA agents and glucocorticoids, although biologics are increasing in popularity. Untreated IBD can lead to severe complications, including fistula formation and malignancy.
Disease pathogenesis for IBD is thought to begin with an inappropriate immune response to endogenous microbiota or other antigens within the intestines (Jameson et al., 2018). Under normal conditions this abnormal response would be quickly suppressed, but aberrant regulation leads to disease. Tregs within the intestines are responsible not only for dampening immune recognition of self-antigens but also for preventing immune responses to harmless dietary and commensal organism antigens (Tanoue et al., 2016). It is unclear what causes the failure of the regulatory systems to ameliorate inflammation in patients with IBD, but an observed decrease in the Treg/Th17 ratio in patient populations is well established (Ueno et al., 2013).
Several preclinical studies have demonstrated the potential utility of ITK blockade in the treatment of IBD (Cho et al., 2015, 2020). These studies showed that ITK-knockout mice have impaired migration of lymphocytes into intestinal tissue (Cho et al., 2020) and that the use of a small molecule inhibitor of ITK in a murine model of IBD decreased symptoms, decreased migration of Th1 and Th17 cells, and paradoxically decreased Treg differentiation (Cho et al., 2015). To our knowledge, no clinical studies have investigated the use of ITK inhibitors in IBD. We believe the evidence warrants further pre-clinical and clinical investigation.
Psoriasis
Psoriasis is one of the most common cutaneous conditions, affecting up to 2% of the worldwide population (Jameson et al., 2018). It is characterized by the presence of scaly plaques commonly located on extensor surfaces, the scalp, and the trunk. Treatment typically consists of topical glucocorticoids, with systemic immunosuppressive agents or targeted biologics reserved for severe disease. The etiology of psoriasis remains elusive, but evidence suggests that it is a T cell-mediated disease with a strong genetic component.
The involvement of IL-23 and Th17 function in psoriasis pathogenesis is well established. IL-23 injection into mice has been shown to mimic psoriasis plaque development (Mak et al., 2009), and genetic studies identified variants of the IL-23 receptor that are protective against psoriasis (Capon et al., 2007). Treg cells have also been shown to play a role in disease pathogenesis, as serum analysis of patients with psoriasis showed adequate levels of Tregs, but these Tregs were dysfunctional in their inhibitory properties (Sugiyama et al., 2005).
Several studies have investigated the role of ITK in psoriasis pathogenesis. One study showed that mRNA expression of ITK, was upregulated in human psoriatic plagues (Fuhriman et al., 2018). The same study found that the use of a small molecule inhibitor of both RTK and ITK decreased cytokine production by Th17 cells in vitro and resulted in a clinical reduction of both scaling and erythema as well as decreased epidermal hyperplasia in two separate psoriatic murine models. Immunofluorescent staining showed a decreased dermal phosphorylation of STAT3 following ITK/RTK blockage, which has been linked to keratinocyte proliferation in psoriasis. Systemic TNF-α levels were also reduced following treatment. A separate study successfully showed that activation of STAT3 and NF-κB by upstream ITK was associated with upregulation of Th17 cells and increased neutrophilic inflammation of the skin in a murine model (Nadeem et al., 2020a). They additionally found that preventative treatment with an ITK inhibitor resulted in a reduction of Th17 cells and enhancement of Tregs. Finally, administration of the first topical ITK inhibitor was shown to be effective in a murine model of psoriasis (Otake et al., 2021).
Solid organ and bone marrow transplantation
Solid organ transplantation
The only treatment of severe end organ damage is organ transplantation (Black et al., 2018). Solid organ transplant recipients must take chronic immunosuppressive medications, such as calcineurin inhibitors, mTOR inhibitors, and glucocorticoids, in order to prevent immune-mediated transplant rejection. The chronic use of these medications can have debilitating side effects, and these drugs are especially nephrotoxic. Graft rejection is mediated by T cells, which recognize allo-antigens, resulting in both cellular and humoral immune responses (Ingulli, 2010).
Tregs suppress the immune functions of other cells through multiple mechanisms, including synthesis of the anti-inflammatory cytokines TGF-β and IL-10, direct cytotoxicity against effector T and B lymphocytes, and possibly even direct suppressive effects against dendritic cells (Vignali et al., 2008). Several clinical and pre-clinical trials have investigated the utility of enhancing Treg function as a replacement for chronic immunosuppression in the solid organ transplant patient population. Phase I trials have shown that autologous Treg transplantation is safe in kidney and liver transplant recipients, although no conclusions on the efficacy of Treg treatment can be made from these studies (Chandran et al., 2017; Mathew et al., 2018; Todo et al., 2016). However, current phase II trials are underway in both liver and kidney transplant recipients (NCT02711826, NCT01446484, NCT01446484).
Despite the lack of clinical evidence for the efficacy of autologous Treg transplantation, other preclinical and clinical studies suggest that upregulation of Treg function increases graft tolerance and prevents rejection (Fisher et al., 2019). However, at least one clinical trial showed that Treg levels did not correlate with lower graft injury in kidney transplant recipients (Ruggenenti et al., 2007). Even if autologous Treg transplantation is proven to be efficacious in preventing graft rejection, its utility will be hindered by the complexity and expense of generating Treg cells ex vivo. It is reasonable to theorize that the inhibition of ITK could mimic the effects of autologous Treg transplantation due to ITK's inhibitory role in FOXP3 expression, thereby ameliorating the necessity for expensive and complicated ex vivo expansion of Tregs. One recent study showed that ITK knockdown using RNA interference in primary human CD4 cells increased the expression of Foxp3, resulting in a subsequent increase in Treg differentiation (Mamontov et al., 2019). Of interest, the opposite was observed in primary human CD4 cells following treatment with an RTK/ITK inhibitor, suggesting that RTK and ITK may have opposing roles in the regulation of Foxp3 expression. Another study showed that ITK inhibition increased allograft survival and reduced Th1/Th17 function in a murine model of cardiac transplantation, although a corresponding increase in Treg function was not observed (Huang et al., 2020) (Figure 2).
Figure 2.
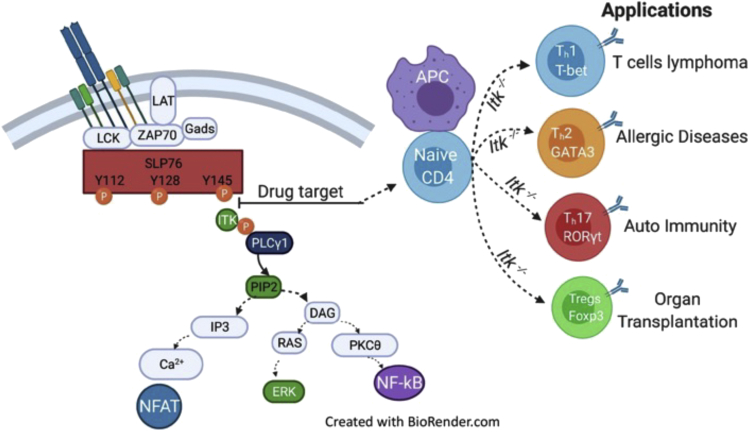
ITK is a critical for several T cells mediated diseases
Selective inhibition of SLP76:ITK signaling will have a significant impact on T cell lymphoma, owing to Th1 cell expansion and increased T-bet and Eomes expression. This inhibition might also have an impact on allergic diseases, owing to Th2 cell reduction and decreased GATA3 expression. ITK inhibition will have an impact on autoimmune diseases owing to Th17 cell reduction and reduced expression of RORγt. The inhibition of ITK upregulates FOXP3-positive Tregs, which are critical for improving organ transplantation outcomes.
Bone marrow transplantation
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a well-established treatment of hematological disorders that are resistant to initial therapy (Copelan et al., 2019). Allo-HSCT involves grafting of hematopoietic stem cells from donors with similar, or in the best-case scenario, identical, HLA alleles. The most common life-threatening complication of such an invasive therapy is graft-versus-host disease (GVHD) (Copelan et al., 2019). Approximately 30%–70% of allo-HSCT recipients will develop acute GVHD, which typically presents with a diffuse maculopapular rash, liver dysfunction, and diarrhea. GVHD is mediated by donor T cells (from the graft), which mount a systemic immune response against the recipient's tissues (the host). Current standard therapy for the prophylaxis of GVHD in allo-HSCT recipients includes the combined use of calcineurin inhibitors and methotrexate (Ruutu et al., 2014). Despite the utility of immunosuppressive agents in preventing GVHD, they also suppress the beneficial phenomenon often seen in allo-HSCT patients known as the graft-versus-leukemia (GVL) effect (Chang et al., 2018). GVL, like GVHD, is mediated by donor T cells, but the donor T cells mount an immune response against malignant cells rather than against normal healthy tissues (Mammadli et al., 2020). However, because GVL and GVHD are mediated by the same donor T cells, these processes are intricately linked (Figure 3).
Figure 3.
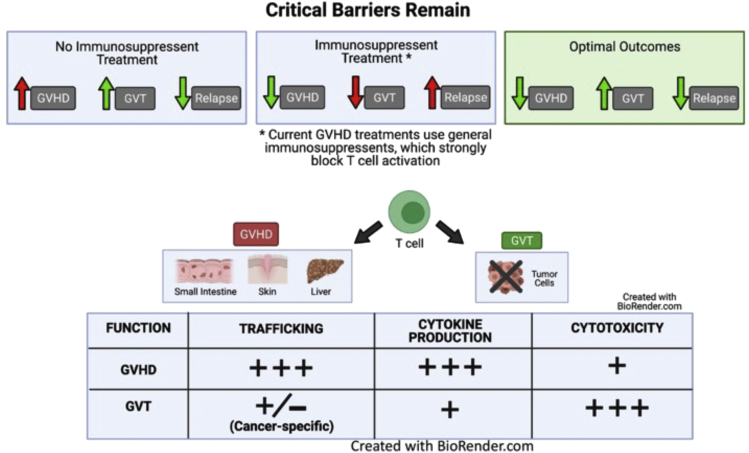
Targeting ITK can differentiate GVHD from CVL
Current treatments to suppress GVHD following allo-HSCT often involve general immunosuppressive agents, such as cyclosporine A, which block donor T cell receptor (TCR)-mediated signaling and hence block general T cell activation. However, these agents can simultaneously inhibit GVL effects, increasing the chance of relapse. Thus, the identification of TCR-mediated signaling pathways that selectively maintain GVL effects while attenuating GVHD would be an optimal therapeutic intervention to differentiate GVHD from GVL. GVHD and GVL are mediated by the same donor T cells. Therefore, it is essential to understand which functions of T cells are critical for GVHD and which are important for GVL function. These donor T cell functions include donor T cell trafficking to GVHD target organs, inflammatory cytokine production by donor T cells, and cytotoxicity against tumor cells.
Our own work and that of others has highlighted some key differences in the pathogenesis of GVL and GVHD, which may be utilized to prevent GVHD without suppressing GVL. GVHD pathogenesis relies heavily on inflammatory cytokine production and T cell migration to target tissues and can be ameliorated by an upregulation of Treg cells, whereas GVL tends to be mediated by direct cell-mediated cytotoxicity (Chang et al., 2018). Therefore, ITK inhibition may be effective in preventing GVHD owing to suppression of pro-inflammatory Th2- and Th17-related cytokines and upregulation of Tregs, while allowing for and possibly enhancing the cell-mediated cytotoxic effects.
Both clinical and preclinical studies have shown the utility of ibrutinib in preventing GVHD. In fact, our laboratory has recently shown that mutation or inhibition of the adaptive linker molecule SLP76, which is essential for ITK signaling, effectively reduced GVHD symptoms without a corresponding suppression of GVL in murine models (Mammadli et al., 2020, 2021a). These studies showed that irradiated mice transplanted with ITK knockout T cells and challenged with B-ALL cells still benefited from GVL but did not suffer from severe GVHD and lived longer post bone marrow transplant than irradiated mice that were transplanted with wild-type T cells. Reduced cytokine production and donor T cell infiltration of the liver, skin, and intestine in irradiated mice that received transplantation with ITK knockout T cells were also observed. These observations were reproduced when irradiated mice were transplanted with T cells with a SLP76 mutation at the Y145 position. Finally, a peptide that inhibits the phosphorylation of SLP76 at the Y145 position was shown to decrease phosphorylation of ITK and PLCγ in both human and mouse T cells in vitro. The SLP76 inhibitor was also successful in uncoupling GVL from GVHD in the same murine model of GVHD (Mammadli et al., 2020, 2021b).
A clinical study showed that none of the 27 patients with CLL observed developed GVHD following allo-HSCT and ibrutinib treatment (Ryan et al., 2016). The study also found a decrease in GATA3 expression and Th2 function with no corresponding effect on T-bet expression following treatment with ibrutinib in the same human sample. Despite these exciting results in human patients, clinical trials comparing the efficacy of the current standard drugs for GVHD prophylaxis to ibrutinib are needed.
Current limitations
To our knowledge, the only completed clinical study investigating the efficaciousness of ITK blockade in patients with T cell lymphoma failed despite the preponderance of pre-clinical evidence suggesting that this blockade would be beneficial (Kumar et al., 2018). However, clinical study cannot definitively disprove the potential benefit of ITK inhibition in T cell malignancy, for several reasons. These include the small sample size (n = 14), the diversity of pathologies treated in the study (both cutaneous T cell lymphoma and various subtypes of PTCL), and that the study only included patients suffering from refractory, previously treated disease. In fact, a larger-scale trial investigating a novel ITK inhibitor is currently underway in patients with T cell lymphoma and is scheduled to be completed in 2022 (NCT03952078).
As previously stated, PTCL is a notoriously difficult disease to categorize owing to its heterogeneity. The elevated expression of ITK and the presence of constitutively active ITK fusion proteins may be limited to specific subtypes of PTCL, rather than being a general characteristic of the disease (Sabattini et al., 2010; Boddicker et al., 2016). Therefore, an improved understanding of the presence of aberrant ITK signaling in various subtypes of PTCL, through more robust observational studies, could facilitate the formation of clinical trials in subpopulations of patients who are most likely to benefit from ITK inhibition.
Observation of the few individuals with confirmed genetic ITK deficiencies allows us to theorize the potential complications of pharmaceutical inhibition of ITK. Case reports of these individuals reveal an increased risk of EBV-induced lymphoproliferative disease, which can progress to lymphoma (Ghosh et al., 2014). Other reports show an inability to mount proper immune responses to human papilloma virus, leading to disseminated cutaneous warts that can develop into squamous cell carcinoma (Youssefian et al., 2019). This is an interesting juxtaposition to other viral infections such as HIV or influenza, which may require the ITK protein for replication within T cells (Fan et al., 2012; Readinger et al., 2008). Opportunistic infections will also be of concern in patients receiving ITK inhibitors. Ibrutinib monotherapy is associated with invasive fungal and bacterial infection in patients with leukemia and lymphoma (Varughese et al., 2018).
Caution must also be used when interpreting the effect of ibrutinib in the pre-clinical and clinical studies mentioned above. B cells are essential for the pathogenesis of many of the diseases discussed, and it is therefore possible that some of the observed benefits of ibrutinib are derived from dual blockage of both ITK and BTK. If the observed efficacy of ibrutinib is due to dual blockage, a more targeted ITK inhibitor may prove to be less efficacious. The expression of BTK and other Tec family kinases by mast cells also potentially confounds observed results (Felices et al., 2007).
Future directions
Further investigation into ITK inhibition as an immunotherapeutic target will be particularly interesting. In fact, several larger-scale clinical trials are currently recruiting for the use of ibrutinib as an adjuvant therapy in patients with CLL (NCT04639362, NCT03400176, NCT04771507). The result of these studies will be particularly interesting, and if proven efficacious, ITK inhibitors could help usher in a new age of immunotherapeutic potential. However, it will be difficult to determine if any observed outcome is primarily from direct inhibition of malignant cells (BTK inhibition) or from a Th1 transformation (ITK inhibition). However, a separate clinical study slated to end in 2021 is investigating how ibrutinib alters the immune cell population in patients with metastatic solid tumors (NCT03525925). The results of this study will better support the utility of ITK inhibitors as an immunotherapeutic target.
Another avenue of study would be to investigate if Fenebrutinib, a BTK inhibitor, which in phase II clinical trials was shown to be efficacious in patients with RA (Cohen et al., 2020), is also an inhibitor of ITK. As previously stated, it is reasonable to theorize that some of the observed benefit in this clinical trial was partially due to ITK inhibition, since to our knowledge, no small molecule has been shown to inhibit ITK or BTK without inhibiting the corresponding homolog. In our laboratory, we are attempting to overcome this homology problem by investigating whether our novel SLP-76 inhibitor is specific and does not inhibit the corresponding B cell homolog, SLP-65, which activates BTK. This research is ongoing, but we believe more investigation into the inhibition of upstream activators of critical signaling kinases, such as ITK and BTK, will prove to be an effective strategy in bypassing the conserved structure seen in many tyrosine kinases.
Conclusion
We hope that this review may facilitate more robust pre-clinical and clinical investigations into the use of ibrutinib and other ITK inhibitors for diverse pathologies. We believe that the pre-clinical and clinical literature strongly supports further investigation into ITK inhibitors for the targeted treatment of the diseases highlighted in this review. Ibrutinib is currently the most feasible drug for clinical use as an ITK inhibitor, since it has been approved by the FDA and has been shown to be efficacious in several of the diseases discussed above. However, ibrutinib's indiscriminate inhibition of both ITK and BTK has the potential to lead to severe side effects such as immunosuppression. Therefore, novel inhibitors that disrupt ITK signaling, such as the SLP76 inhibitor previously described (Mammadli et al., 2021b), are needed in order to effectively overcome the problem of ITK/BTK homology, and will thus be a more targeted and effective therapy. We must additionally acknowledge that some of the observed benefit of ibrutinib in the various autoimmune and allergic conditions discussed could be due to its inhibitory effect on B cells and other immune cells and not solely due to ITK inhibition within T cells.
Limitations of the study
Currently, this study's limitation is that our novel peptide SLP76pTYR can only be used in vitro or as a construct to transduce T cells. We are working with structure and medicinal chemist to make our peptide smaller and stable to be used in in vivo studies. But in in vitro studies, our peptide reproduces the SLP76Y145FKI mice
Table 1.
The role of ITK in various diseases, with supporting references
| Diseases | Current treatment | The role of ITK | Possible therapy | References |
|---|---|---|---|---|
| Peripheral T cell lymphoma |
Chemotherapy regimen | ITK-SYK fusion protein | ITK and BTK Inhibitors |
Sabattini et al., 2010 Moskowitz et al., 2014 Moskowitz et al., 2014 Li et al., 2020 |
| Asthma | Inhaled corticosteroids | ITK polymorphisms | ITK and BTK Inhibitors |
Mueller and August, 2003 Lee et al., 2011 Sun et al., 2015 |
| Atopic dermatitis | Glucocorticoids | ITK polymorphisms | ITK and BTK Inhibitors |
Graves et al., 2005 Benson et al., 2009 Matsumoto et al., 2002 |
| Leishmaniasis | Pentostam Glucantime |
Regulation of GATA-3 | ITK and BTK Inhibitors |
Graves et al., 2005 Dubovsky et al., 2013 Varikuti et al., 2019 |
| Listeria monocytogenes | Antibiotic therapy | Th2 cytokine | ITK and BTK Inhibitors |
Truong and Ashurst, 2021 |
| Pneumocystis pneumonia (PCP) | Antibiotic prophylaxis | Th2 cytokine | Selective ITK Inhibitors | Dubovsky et al., 2013 |
| HIV | Antiretroviral therapy | Actin polymerization Nef activates ITK |
Selective ITK Inhibitors |
Hain et al., 2018 Tarafdar et al., 2014 Basmaciogullari and Pizzato, 2014 |
| Rheumatoid arthritis (RA) | Chemotherapeutic, methotrexate | ITK regulates the Th17 cells | ITK and BTK Inhibitors |
Raza et al., 2005 Zizzo et al., 2011 Gomez-Rodriguez et al., 2009 |
| Systemic lupus erythematosus (SLE) | Hydroxychloroquine Glucocorticoids |
Th17 increase Treg decrease | ITK and BTK Inhibitors |
Gomez-Rodriguez et al., 2009, Nadeem et al., 2020b |
| Multiple sclerosis (MS) | Glucocorticoids Natalizumab |
Th1- and Th17-related cytokines | ITK and BTK Inhibitors |
Jameson et al., 2018 Havrdová et al., 2016 |
| Inflammatory bowel disease (IBD | 5-ASA glucocorticoids | Decrease in the Treg/Th17 ratio | Selective ITK Inhibitors |
Ueno et al., 2013 Cho et al., 2020 |
| Psoriasis | Glucocorticoids Immunosuppressive agents |
IL-23 and Th17 function | Selective ITK Inhibitors |
Mak et al., 2009 Capon et al., 2007 Sugiyama et al., 2005 |
| Solid organ transplantation | Glucocorticoids Calcineurin inhibitors mTOR inhibitors |
Th1/Th17 cytokines | Selective ITK inhibitors reduced cytokine |
Ingulli, 2010 Huang et al., 2020 Fisher et al., 2019 |
| Bone marrow transplantation | Calcineurin inhibitors methotrexate Glucocorticoids |
Th1/Th17 cytokines | Selective ITK Inhibitors |
Chang et al., 2018 Mammadli et al., 2020 |
.
Acknowledgments
We thank all members of the Karimi laboratories for helpful discussions. This research was funded in part by a grant from the National Blood Foundation Scholar Award to (M.K.) and the National Institutes of Health (NIH LRP #L6 MD0010106 and K22 (AI130182) to M.K. Upstate Medical University Cancer grant (1146249-1-75632) to M.K. The figures for this review were made using Biorender.com.
Author contributions
S.W., R.H., and M.K. analyzed the data and wrote the manuscript.
Declaration of interests
The authors declare no conflicts of interest.
References
- Agostinelli C., Rizvi H., Paterson J., Shende V., Akarca A.U., Agostini E., Fuligni F., Righi S., Spagnolo S., Piccaluga P.P. Intracellular TCR-signaling pathway: novel markers for lymphoma diagnosis and potential therapeutic targets. Am. J. Surg. Pathol. 2014;38:1349–1359. doi: 10.1097/PAS.0000000000000309. [DOI] [PubMed] [Google Scholar]
- Allchin R.L., Kelly M.E., Mamand S., Doran A.G., Keane T., Ahearne M.J., Wagner S.D. Structural and diffusion weighted MRI demonstrates responses to ibrutinib in a mouse model of follicular helper (Tfh) T-cell lymphoma. PLoS One. 2019;14:e0215765. doi: 10.1371/journal.pone.0215765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreotti A.H., Schwartzberg P.L., Joseph R.E., Berg L.J. T-cell signaling regulated by the Tec family kinase, Itk. Cold Spring Harb. Perspect. Biol. 2010;2:a002287. doi: 10.1101/cshperspect.a002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basmaciogullari S., Pizzato M. The activity of Nef on HIV-1 infectivity. Front Microbiol. 2014;5:232. doi: 10.3389/fmicb.2014.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M., Mobini R., Barrenäs F., Halldén C., Naluai A.T., Säll T., Cardell L.O. A haplotype in the inducible T-cell tyrosine kinase is a risk factor for seasonal allergic rhinitis. Allergy. 2009;64:1286–1291. doi: 10.1111/j.1398-9995.2009.01991.x. [DOI] [PubMed] [Google Scholar]
- Berg L.J., Finkelstein L.D., Lucas J.A., Schwartzberg P.L. Tec family kinases in T lymphocyte development and function. Annu. Rev. Immunol. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- Black C.K., Termanini K.M., Aguirre O., Hawksworth J.S., Sosin M. Solid organ transplantation in the 21(st) century. Ann. Transl. Med. 2018;6:409. doi: 10.21037/atm.2018.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco F.J., Möricke R., Dokoupilova E., Codding C., Neal J., Andersson M., Rohrer S., Richards H. Secukinumab in active rheumatoid arthritis: a phase III randomized, double-blind, active comparator- and placebo-controlled study. Arthritis Rheumatol. 2017;69:1144–1153. doi: 10.1002/art.40070. [DOI] [PubMed] [Google Scholar]
- Boddicker R.L., Razidlo G.L., Dasari S., Zeng Y., Hu G., Knudson R.A., Greipp P.T., Davila J.I., Johnson S.H., Porcher J.C. Integrated mate-pair and RNA sequencing identifies novel, targetable gene fusions in peripheral T-cell lymphoma. Blood. 2016;128:1234–1245. doi: 10.1182/blood-2016-03-707141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnell S.C., Diehn M., Yaffe M.B., Findell P.R., Cantley L.C., Berg L.J. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J. Biol. Chem. 2000;275:2219–2230. doi: 10.1074/jbc.275.3.2219. [DOI] [PubMed] [Google Scholar]
- Capon F., Di Meglio P., Szaub J., Prescott N.J., Dunster C., Baumber L., Timms K., Gutin A., Abkevic V., Burden A.D. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum. Genet. 2007;122:201–206. doi: 10.1007/s00439-007-0397-0. [DOI] [PubMed] [Google Scholar]
- Chandran S., Tang Q., Sarwal M., Laszik Z.G., Putnam A.L., Lee K., Leung J., Nguyen V., Sigdel T., Tavares E.C. Polyclonal regulatory T cell therapy for control of inflammation in kidney transplants. Am. J. Transplant. 2017;17:2945–2954. doi: 10.1111/ajt.14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.J., Zhao X.Y., Huang X.J. Strategies for enhancing and preserving anti-leukemia effects without aggravating graft-versus-host disease. Front Immunol. 2018;9:3041. doi: 10.3389/fimmu.2018.03041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.S., Ha S., Shin H.M., Reboldi A., Hall J.A., Huh J.R., Usherwood E.J., Berg L.J. CD8(+) T cells require ITK-mediated TCR signaling for migration to the intestine. Immunohorizons. 2020;4:57–71. doi: 10.4049/immunohorizons.1900093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.S., Shin H.M., Haberstock-Debic H., Xing Y., Owens T.D., Funk J.O., Hill R.J., Bradshaw J.M., Berg L.J. A small molecule inhibitor of ITK and RLK impairs Th1 differentiation and prevents colitis disease progression. J. Immunol. 2015;195:4822–4831. doi: 10.4049/jimmunol.1501828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Tuckwell K., Katsumoto T.R., Zhao R., Galanter J., Lee C., Rae J., Toth B., Ramamoorthi N., Hackney J.A. Fenebrutinib versus placebo or adalimumab in rheumatoid arthritis: a randomized, double-blind, phase II trial (ANDES study) Arthritis Rheumatol. 2020;72:1435–1446. doi: 10.1002/art.41275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copelan E.A., Chojecki A., Lazarus H.M., Avalos B.R. Allogeneic hematopoietic cell transplantation; the current renaissance. Blood Rev. 2019;34:34–44. doi: 10.1016/j.blre.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Dall'Era M., Pauli M.L., Remedios K., Taravati K., Sandova P.M., Putnam A.L., Lares A., Haemel A., Tang Q., Hellerstein M. Adoptive treg cell therapy in a patient with systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:431–440. doi: 10.1002/art.40737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayakar A., Chandrasekaran S., Kuchipudi S.V., Kalangi S.K. Cytokines: key determinants of resistance or disease progression in visceral leishmaniasis: opportunities for novel diagnostics and immunotherapy. Front Immunol. 2019;10:670. doi: 10.3389/fimmu.2019.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovsky J.A., Beckwith K.A., Natarajan G., Woyach J.A., Jaglowski S., Zhong Y., Hessler J.D., Liu T.M., Chang B.Y., Larkin K.M. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–2549. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eken A., Cansever M., Somekh I., Mizoguchi Y., Zietara N., Okus F.Z., Erdem S., Canatan H., Akyol S., Ozcan A. Genetic deficiency and biochemical inhibition of ITK affect human Th17, Treg, and innate lymphoid cells. J. Clin. Immunol. 2019;39:391–400. doi: 10.1007/s10875-019-00632-5. [DOI] [PubMed] [Google Scholar]
- Elmore J.P., Mcgee M.C., Nidetz N.F., Anannya O., Huang W., August A. Tuning T helper cell differentiation by ITK. Biochem. Soc. Trans. 2020;48:179–185. doi: 10.1042/BST20190486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F., Yoo H.J., Stock S., Wang L., Liu Y., Schubert M.L., Wang S., Neuber B., Hückelhoven-Krauss A., Gern U. Ibrutinib for improved chimeric antigen receptor T-cell production for chronic lymphocytic leukemia patients. Int. J. Cancer. 2021;148:419–428. doi: 10.1002/ijc.33212. [DOI] [PubMed] [Google Scholar]
- Fan K., Jia Y., Wang S., Li H., Wu D., Wang G., Chen J.L. Role of Itk signalling in the interaction between influenza A virus and T-cells. J. Gen. Virol. 2012;93:987–997. doi: 10.1099/vir.0.041228-0. [DOI] [PubMed] [Google Scholar]
- Fang N., Motto D.G., Ross S.E., Koretzky G.A. Tyrosines 113, 128, and 145 of SLP-76 are required for optimal augmentation of NFAT promoter activity. J. Immunol. 1996;157:3769–3773. [PubMed] [Google Scholar]
- Fasching P., Stradner M., Graninger W., Dejaco C., Fessler J. Therapeutic potential of targeting the Th17/Treg axis in autoimmune disorders. Molecules. 2017;22 doi: 10.3390/molecules22010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felices M., Falk M., Kosaka Y., Berg L.J. Tec kinases in T cell and mast cell signaling. Adv. Immunol. 2007;93:145–184. doi: 10.1016/S0065-2776(06)93004-1. [DOI] [PubMed] [Google Scholar]
- Ferrara T.J., Mueller C., Sahu N., Ben-Jebria A., August A. Reduced airway hyperresponsiveness and tracheal responses during allergic asthma in mice lacking tyrosine kinase inducible T-cell kinase. J. Allergy Clin. Immunol. 2006;117:780–786. doi: 10.1016/j.jaci.2005.12.1330. [DOI] [PubMed] [Google Scholar]
- Fisher J.D., Balmert S.C., Zhang W., Schweizer R., Schnider J.T., Komatsu C., Dong L., Erbas V.E., Unadkat J.V., Aral A.M. Treg-inducing microparticles promote donor-specific tolerance in experimental vascularized composite allotransplantation. Proc. Natl. Acad. Sci. U S A. 2019;116:25784–25789. doi: 10.1073/pnas.1910701116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowell D.J., Shinkai K., Liao X.C., Beebe A.M., Coffman R.L., Littman D.R., Locksley R.M. Impaired NFATc translocation and failure of Th2 development in Itk-deficient CD4+ T cells. Immunity. 1999;11:399–409. doi: 10.1016/s1074-7613(00)80115-6. [DOI] [PubMed] [Google Scholar]
- Fuhriman J.M., Winge M.C.G., Haberstock-Debic H., Funk J.O., Bradshaw J.M., Marinkovich M.P. ITK and RLK inhibitor PRN694 improves skin disease in two mouse models of psoriasis. J. Invest. Dermatol. 2018;138:864–871. doi: 10.1016/j.jid.2017.10.029. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Bienemann K., Boztug K., Borkhardt A. Interleukin-2-inducible T-cell kinase (ITK) deficiency - clinical and molecular aspects. J. Clin. Immunol. 2014;34:892–899. doi: 10.1007/s10875-014-0110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rodriguez J., Meylan F., Handon R., Hayes E.T., Anderson S.M., Kirby M.R., Siegel R.M., Schwartzberg P.L. Itk is required for Th9 differentiation via TCR-mediated induction of IL-2 and IRF4. Nat. Commun. 2016;7:10857. doi: 10.1038/ncomms10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rodriguez J., Sahu N., Handon R., Davidson T.S., Anderson S.M., Kirby M.R., August A., Schwartzberg P.L. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity. 2009;31:587–597. doi: 10.1016/j.immuni.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rodriguez J., Wohlfert E.A., Handon R., Meylan F., Wu J.Z., Anderson S.M., Kirby M.R., Belkaid Y., Schwartzberg P.L. Itk-mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. J. Exp. Med. 2014;211:529–543. doi: 10.1084/jem.20131459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves P.E., Siroux V., Guerra S., Klimecki W.T., Martinez F.D. Association of atopy and eczema with polymorphisms in T-cell immunoglobulin domain and mucin domain-IL-2-inducible T-cell kinase gene cluster in chromosome 5 q 33. J. Allergy Clin. Immunol. 2005;116:650–656. doi: 10.1016/j.jaci.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Hain A., Krämer M., Linka R.M., Nakhaei-Rad S., Ahmadian M.R., Häussinger D., Borkhardt A., Münk C. IL-2 inducible kinase ITK is critical for HIV-1 infection of Jurkat T-cells. Sci. Rep. 2018;8:3217. doi: 10.1038/s41598-018-21344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselmayer P., Camps M., Liu-Bujalski L., Nguyen N., Morandi F., Head J., O'Mahony A., Zimmerli S.C., Bruns L., Bender A.T. Efficacy and pharmacodynamic modeling of the BTK inhibitor evobrutinib in autoimmune disease models. J. Immunol. 2019;202:2888–2906. doi: 10.4049/jimmunol.1800583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havrdová E., Belova A., Goloborodko A., Tisserant A., Wright A., Wallstroem E., Garren H., Maguire R.P., Johns D.R. Activity of secukinumab, an anti-IL-17A antibody, on brain lesions in RRMS: results from a randomized, proof-of-concept study. J. Neurol. 2016;263:1287–1295. doi: 10.1007/s00415-016-8128-x. [DOI] [PubMed] [Google Scholar]
- Hewitt S.L., Bailey D., Zielinski J., Apte A., Musenge F., Karp R., Burke S., Garcon F., Mishra A., Gurumurthy S. Intratumoral IL12 mRNA therapy promotes TH1 transformation of the tumor microenvironment. Clin. Cancer Res. 2020;26:6284–6298. doi: 10.1158/1078-0432.CCR-20-0472. [DOI] [PubMed] [Google Scholar]
- Huang Y., Wang Z., Li H., Hu Z., Hong H., Sun Y., Ke Y., Du X. ITK inhibition promotes long-term survival of cardiac allografts by regulating T cell PLCγ phosphorylation. Am. J. Transl Res. 2020;12:5762–5771. [PMC free article] [PubMed] [Google Scholar]
- Ingulli E. Mechanism of cellular rejection in transplantation. Pediatr. Nephrol. 2010;25:61–74. doi: 10.1007/s00467-008-1020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson F., Kasper, Hauser, Longo, Loscalzo . McGraw-Hill Education; 2018. Harrison's Principles of Internal Medicine. [Google Scholar]
- Jordan M.S., Sadler J., Austin J.E., Finkelstein L.D., Singer A.L., Schwartzberg P.L., Koretzky G.A. Functional hierarchy of the N-terminal tyrosines of SLP-76. J. Immunol. 2006;176:2430–2438. doi: 10.4049/jimmunol.176.4.2430. [DOI] [PubMed] [Google Scholar]
- Kannan A.K., Kim D.G., August A., Bynoe M.S. Itk signals promote neuroinflammation by regulating CD4+ T-cell activation and trafficking. J. Neurosci. 2015;35:221–233. doi: 10.1523/JNEUROSCI.1957-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan A.K., Mohinta S., Huang W., Huang L., Koylass N., Appleton J.A., August A. T-Bet independent development of IFNγ secreting natural T helper 1 cell population in the absence of Itk. Sci. Rep. 2017;7:45935. doi: 10.1038/srep45935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Perl A. Blockade of Treg cell differentiation and function by the interleukin-21-mechanistic target of rapamycin axis via suppression of autophagy in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2018;70:427–438. doi: 10.1002/art.40380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kim B.E., Leung D.Y.M. Pathophysiology of atopic dermatitis: clinical implications. Allergy Asthma Proc. 2019;40:84–92. doi: 10.2500/aap.2019.40.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.H., Liu H.L., Mraz-Gernhard S., Varghese A., Hoppe R.T. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch. Dermatol. 2003;139:857–866. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- Kumar A., Vardhana S., Moskowitz A.J., Porcu P., Dogan A., Dubovsky J.A., Matasar M.J., Zhang Z., Younes A., Horwitz S.M. Pilot trial of ibrutinib in patients with relapsed or refractory T-cell lymphoma. Blood Adv. 2018;2:871–876. doi: 10.1182/bloodadvances.2017011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner K.S., Neurath M.F., Weigmann B. Role of the IL-2 inducible tyrosine kinase ITK and its inhibitors in disease pathogenesis. J. Mol. Med. (Berl) 2020;98:1385–1395. doi: 10.1007/s00109-020-01958-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G.R. The balance of Th17 versus Treg cells in autoimmunity. Int. J. Mol. Sci. 2018;19:730. doi: 10.3390/ijms19030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Chang H.S., Jang A.S., Park S.W., Park J.S., Uh S.T., Kim Y.H., Oh B., Lee J.K., Park B.L. The association of a single-nucleotide polymorphism of the IL-2 inducible T-cell Kinase gene with asthma. Ann. Hum. Genet. 2011;75:359–369. doi: 10.1111/j.1469-1809.2010.00637.x. [DOI] [PubMed] [Google Scholar]
- Li H., Wang C., Ma P., Zhang M., Yang H., Yuan S., Wei J., Tao L., Qian K., Xu M., Li L. The retinoid derivant ECPIRM selectively exhibited anti-proliferation effects in cutaneous T-Cell lymphoma via ITK-mediated signaling pathway. J. Dermatol. Sci. 2020;97:208–215. doi: 10.1016/j.jdermsci.2020.01.013. [DOI] [PubMed] [Google Scholar]
- Liang P.I., Chang S.T., Lin M.Y., Hsieh Y.C., Chu P.Y., Chen C.J., Lin K.J., Jung Y.C., Hwang W.S., Huang W.T. Angioimmunoblastic T-cell lymphoma in Taiwan shows a frequent gain of ITK gene. Int. J. Clin. Exp. Pathol. 2014;7:6097–6107. [PMC free article] [PubMed] [Google Scholar]
- Liao X.C., Littman D.R. Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity. 1995;3:757–769. doi: 10.1016/1074-7613(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Lin T.A., Mcintyre K.W., Das J., Liu C., O'Day K.D., Penhallow B., Hung C.Y., Whitney G.S., Shuster D.J., Yang X. Selective Itk inhibitors block T-cell activation and murine lung inflammation. Biochemistry. 2004;43:11056–11062. doi: 10.1021/bi049428r. [DOI] [PubMed] [Google Scholar]
- Liu K.Q., Bunnell S.C., Gurniak C.B., Berg L.J. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J. Exp. Med. 1998;187:1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang X., Deng L., Ping L., Shi Y., Zheng W., Lin N., Wang X., Tu M., Xie Y. ITK inhibition induced in vitro and in vivo anti-tumor activity through downregulating TCR signaling pathway in malignant T cell lymphoma. Cancer Cell Int. 2019;19:32. doi: 10.1186/s12935-019-0754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M., Beckwith K., Do P., Mundy B.L., Gordon A., Lehman A.M., Maddocks K.J., Cheney C., Jones J.A., Flynn J.M. Ibrutinib treatment improves T cell number and function in CLL patients. J. Clin. Invest. 2017;127:3052–3064. doi: 10.1172/JCI89756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak R.K., Hundhausen C., Nestle F.O. Progress in understanding the immunopathogenesis of psoriasis. Actas Dermosifiliogr. 2009;100(Suppl 2):2–13. doi: 10.1016/s0001-7310(09)73372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammadli M., Huang W., Harris R., Sultana A., Cheng Y., Tong W., Pu J., Gentile T., Dsouza S., Yang Q. Targeting interleukin-2-inducible T-cell kinase (ITK) differentiates GVL and GVHD in allo-HSCT. Front Immunol. 2020;11:593863. doi: 10.3389/fimmu.2020.593863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammadli M., Huang W., Harris R., Xiong H., Weeks S., May A., Gentile T., Henty-Ridilla J., Waickman A.T., August A. Targeting SLP76: ITK interaction separates GVHD from GVL in allo-HSCT. iScience. 2021;24:102286. doi: 10.1016/j.isci.2021.102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammadli M., Huang W., Harris R., Xiong H., Weeks S., May A., Gentile T., Henty-Ridilla J., Waickman A.T., August A. Targeting SLP76:ITK interaction separates GVHD from GVL in allo-HSCT. iScience. 2021;24:102286. doi: 10.1016/j.isci.2021.102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamontov P., Eberwine R.A., Perrigoue J., Das A., Friedman J.R., Mora J.R. A negative role for the interleukin-2-inducible T-cell kinase (ITK) in human Foxp3+ TREG differentiation. PLoS One. 2019;14:e0215963. doi: 10.1371/journal.pone.0215963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew J.M., J H.V., Lefever A., Konieczna I., Stratton C., He J., Huang X., Gallon L., Skaro A., Ansari M.J., Leventhal J.R. A phase I clinical trial with ex vivo expanded recipient regulatory T cells in living donor kidney transplants. Sci. Rep. 2018;8:7428. doi: 10.1038/s41598-018-25574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Oshida T., Obayashi I., Imai Y., Matsui K., Yoshida N.L., Nagata N., Ogawa K., Obayashi M., Kashiwabara T. Identification of highly expressed genes in peripheral blood T cells from patients with atopic dermatitis. Int. Arch. Allergy Immunol. 2002;129:327–340. doi: 10.1159/000067589. [DOI] [PubMed] [Google Scholar]
- Miller A.T., Wilcox H.M., Lai Z., Berg L.J. Signaling through Itk promotes T helper 2 differentiation via negative regulation of T-bet. Immunity. 2004;21:67–80. doi: 10.1016/j.immuni.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Morrison V.A. Infectious complications of chronic lymphocytic leukaemia: pathogenesis, spectrum of infection, preventive approaches. Best Pract. Res. Clin. Haematol. 2010;23:145–153. doi: 10.1016/j.beha.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Moskowitz A.J., Lunning M.A., Horwitz S.M. How I treat the peripheral T-cell lymphomas. Blood. 2014;123:2636–2644. doi: 10.1182/blood-2013-12-516245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C., August A. Attenuation of immunological symptoms of allergic asthma in mice lacking the tyrosine kinase ITK. J. Immunol. 2003;170:5056–5063. doi: 10.4049/jimmunol.170.10.5056. [DOI] [PubMed] [Google Scholar]
- Nadeem A., Ahmad S.F., Al-Harbi N.O., Ibrahim K.E., Alqahtani F., As Sobeai H.M., Alotaibi M.R. Inhibition of interleukin-2-inducible T-cell kinase causes reduction in imiquimod-induced psoriasiform inflammation through reduction of Th17 cells and enhancement of Treg cells in mice. Biochimie. 2020;179:146–156. doi: 10.1016/j.biochi.2020.09.023. [DOI] [PubMed] [Google Scholar]
- Nadeem A., Ahmad S.F., Al-Harbi N.O., Ibrahim K.E., Siddiqui N., Al-Harbi M.M., Attia S.M., Bakheet S.A. Inhibition of Bruton's tyrosine kinase and IL-2 inducible T-cell kinase suppresses both neutrophilic and eosinophilic airway inflammation in a cockroach allergen extract-induced mixed granulocytic mouse model of asthma using preventative and therapeutic strategy. Pharmacol. Res. 2019;148:104441. doi: 10.1016/j.phrs.2019.104441. [DOI] [PubMed] [Google Scholar]
- Nadeem A., Al-Harbi N.O., Ahmad S.F., Al-Harbi M.M., Alhamed A.S., Alfardan A.S., Assiri M.A., Ibrahim K.E., Albassam H. Blockade of interleukin-2-inducible T-cell kinase signaling attenuates acute lung injury in mice through adjustment of pulmonary Th17/Treg immune responses and reduction of oxidative stress. Int. Immunopharmacol. 2020;83:106369. doi: 10.1016/j.intimp.2020.106369. [DOI] [PubMed] [Google Scholar]
- Otake S., Otsubaki T., Uesato N., Ueda Y., Murayama T., Hayashi M. Topical application of BMS-509744, a selective inhibitor of interleukin-2-inducible T cell kinase, ameliorates imiquimod-induced skin inflammation in mice. Biol. Pharm. Bull. 2021;44:528–534. doi: 10.1248/bpb.b20-00850. [DOI] [PubMed] [Google Scholar]
- Papi A., Brightling C., Pedersen S.E., Reddel H.K. Asthma. Lancet. 2018;391:783–800. doi: 10.1016/S0140-6736(17)33311-1. [DOI] [PubMed] [Google Scholar]
- Pechloff K., Holch J., Ferch U., Schweneker M., Brunner K., Kremer M., Sparwasser T., Quintanilla-Martinez L., Zimber-Strobl U., Streubel B. The fusion kinase ITK-SYK mimics a T cell receptor signal and drives oncogenesis in conditional mouse models of peripheral T cell lymphoma. J. Exp. Med. 2010;207:1031–1044. doi: 10.1084/jem.20092042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q., Sahu N., August A. Tec kinase Itk forms membrane clusters specifically in the vicinity of recruiting receptors. J. Biol. Chem. 2006;281:38529–38534. doi: 10.1074/jbc.M609180200. [DOI] [PubMed] [Google Scholar]
- Rasmussen T.K., Andersen T., Hvid M., Hetland M.L., Hørslev-Petersen K., Stengaard-Pedersen K., Holm C.K., Deleuran B. Increased interleukin 21 (IL-21) and IL-23 are associated with increased disease activity and with radiographic status in patients with early rheumatoid arthritis. J. Rheumatol. 2010;37:2014–2020. doi: 10.3899/jrheum.100259. [DOI] [PubMed] [Google Scholar]
- Raza K., Falciani F., Curnow S.J., Ross E.J., Lee C.Y., Akbar A.N., Lord J.M., Gordon C., Buckley C.D., Salmon M. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res. Ther. 2005;7:R784–R795. doi: 10.1186/ar1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readinger J.A., Schiralli G.M., Jiang J.K., Thomas C.J., August A., Henderson A.J., Schwartzberg P.L. Selective targeting of ITK blocks multiple steps of HIV replication. Proc. Natl. Acad. Sci. U S A. 2008;105:6684–6689. doi: 10.1073/pnas.0709659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggenenti P., Perico N., Gotti E., Cravedi P., D'Agati V., Gagliardini E., Abbate M., Gaspari F., Cattaneo D., Noris M. Sirolimus versus cyclosporine therapy increases circulating regulatory T cells, but does not protect renal transplant patients given alemtuzumab induction from chronic allograft injury. Transplantation. 2007;84:956–964. doi: 10.1097/01.tp.0000284808.28353.2c. [DOI] [PubMed] [Google Scholar]
- Ruutu T., Gratwohl A., De Witte T., Afanasyev B., Apperley J., Bacigalupo A., Dazzi F., Dreger P., Duarte R., Finke J. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transplant. 2014;49:168–173. doi: 10.1038/bmt.2013.107. [DOI] [PubMed] [Google Scholar]
- Ryan C.E., Sahaf B., Logan A.C., O'Brien S., Byrd J.C., Hillmen P., Brown J.R., Dyer M.J., Mato A.R., Keating M.J. Ibrutinib efficacy and tolerability in patients with relapsed chronic lymphocytic leukemia following allogeneic HCT. Blood. 2016;128:2899–2908. doi: 10.1182/blood-2016-06-715284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabattini E., Bacci F., Sagramoso C., Pileri S.A. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102:83–87. [PubMed] [Google Scholar]
- Sagiv-Barfi I., Kohrt H.E., Czerwinski D.K., Ng P.P., Chang B.Y., Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc. Natl. Acad. Sci. U S A. 2015;112:E966–E972. doi: 10.1073/pnas.1500712112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer E.M., Yap G.S., Lewis C.M., Czar M.J., Mcvicar D.W., Cheever A.W., Sher A., Schwartzberg P.L. Mutation of Tec family kinases alters T helper cell differentiation. Nat. Immunol. 2001;2:1183–1188. doi: 10.1038/ni734. [DOI] [PubMed] [Google Scholar]
- Streubel B., Vinatzer U., Willheim M., Raderer M., Chott A. Novel t(5;9)(q33;q22) fuses ITK to SYK in unspecified peripheral T-cell lymphoma. Leukemia. 2006;20:313–318. doi: 10.1038/sj.leu.2404045. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Gyulai R., Toichi E., Garaczi E., Shimada S., Stevens S.R., Mccormick T.S., Cooper K.D. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J. Immunol. 2005;174:164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Peng I., Webster J.D., Suto E., Lesch J., Wu X., Senger K., Francis G., Barrett K., Collier J.L. Inhibition of the kinase ITK in a mouse model of asthma reduces cell death and fails to inhibit the inflammatory response. Sci. Signal. 2015;8:ra122. doi: 10.1126/scisignal.aab0949. [DOI] [PubMed] [Google Scholar]
- Tanoue T., Atarashi K., Honda K. Development and maintenance of intestinal regulatory T cells. Nat. Rev. Immunol. 2016;16:295–309. doi: 10.1038/nri.2016.36. [DOI] [PubMed] [Google Scholar]
- Tarafdar S., Poe J.A., Smithgall T.E. The accessory factor Nef links HIV-1 to Tec/Btk kinases in an Src homology 3 domain-dependent manner. J. Biol. Chem. 2014;289:15718–15728. doi: 10.1074/jbc.M114.572099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todo S., Yamashita K., Goto R., Zaitsu M., Nagatsu A., Oura T., Watanabe M., Aoyagi T., Suzuki T., Shimamura T. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64:632–643. doi: 10.1002/hep.28459. [DOI] [PubMed] [Google Scholar]
- Truong J., Ashurst J.V. StatPearls; 2021. Pneumocystis Jirovecii Pneumonia. [PubMed] [Google Scholar]
- Ueno A., Jijon H., Chan R., Ford K., Hirota C., Kaplan G.G., Beck P.L., Iacucci M., Fort Gasia M., Barkema H.W. Increased prevalence of circulating novel IL-17 secreting Foxp3 expressing CD4+ T cells and defective suppressive function of circulating Foxp3+ regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients. Inflamm. Bowel Dis. 2013;19:2522–2534. doi: 10.1097/MIB.0b013e3182a85709. [DOI] [PubMed] [Google Scholar]
- Varikuti S., Volpedo G., Saljoughian N., Hamza O.M., Halsey G., Ryan N.M., Sedmak B.E., Seidler G.R., Papenfuss T.L., Oghumu S., Satoskar A.R. The potent ITK/BTK inhibitor ibrutinib is effective for the treatment of experimental visceral leishmaniasis Caused by Leishmania donovani. J. Infect Dis. 2019;219:599–608. doi: 10.1093/infdis/jiy552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varughese T., Taur Y., Cohen N., Palomba M.L., Seo S.K., Hohl T.M., Redelman-Sidi G. Serious infections in patients receiving ibrutinib for treatment of lymphoid cancer. Clin. Infect Dis. 2018;67:687–692. doi: 10.1093/cid/ciy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali D.A., Collison L.W., Workman C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonin A., Rausch A., Mengel A., Hitchcock M., Krüger M., Von Ahsen O., Merz C., Röse L., Stock C., Martin S.F. Inhibition of the IL-2-inducible tyrosine kinase (Itk) activity: a new concept for the therapy of inflammatory skin diseases. Exp. Dermatol. 2011;20:41–47. doi: 10.1111/j.1600-0625.2010.01198.x. [DOI] [PubMed] [Google Scholar]
- Vose J., Armitage J., Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J. Clin. Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- Wang D., Huang S., Yuan X., Liang J., Xu R., Yao G., Feng X., Sun L. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell. Mol. Immunol. 2017;14:423–431. doi: 10.1038/cmi.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Lu Y., Polk A., Chowdhury P., Murga-Zamalloa C., Fujiwara H., Suemori K., Beyersdorf N., Hristov A.C., Lim M.S. T-cell receptor signaling activates an ITK/NF-κB/GATA-3 axis in T-cell lymphomas facilitating resistance to chemotherapy. Clin. Cancer Res. 2017;23:2506–2515. doi: 10.1158/1078-0432.CCR-16-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chen S., Chen J., Xie X., Gao S., Zhang C., Zhou S., Wang J., Mai R., Lin Q. Germline genetic patterns underlying familial rheumatoid arthritis, systemic lupus erythematosus and primary Sjogren's syndrome highlight T cell-initiated autoimmunity. Ann. Rheum. Dis. 2020;79:268–275. doi: 10.1136/annrheumdis-2019-215533. [DOI] [PubMed] [Google Scholar]
- Wen S.R., Liu G.J., Feng R.N., Gong F.C., Zhong H., Duan S.R., Bi S. Increased levels of IL-23 and osteopontin in serum and cerebrospinal fluid of multiple sclerosis patients. J. Neuroimmunol. 2012;244:94–96. doi: 10.1016/j.jneuroim.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Xu W.D., Su L.C., Xie Q.B., Zhao Y., Liu Y. Interleukin-2-inducible T-cell kinase expression and relation to disease severity in systemic lupus erythematosus. Clin. Chim. Acta. 2016;463:11–17. doi: 10.1016/j.cca.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Youssefian L., Vahidnezhad H., Yousefi M., Saeidian A.H., Azizpour A., Touati A., Nikbakht N., Hesari K.K., Adib-Sereshki M.M., Zeinali S. Inherited interleukin 2-inducible T-cell (ITK) kinase deficiency in siblings with epidermodysplasia verruciformis and Hodgkin lymphoma. Clin. Infect Dis. 2019;68:1938–1941. doi: 10.1093/cid/ciy942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Liu J., Ge S., Chen C., Li S., Wu X., Feng X., Wang Y., Cai D. Saikosaponin A inhibits breast cancer by regulating Th1/Th2 balance. Front Pharmacol. 2019;10:624. doi: 10.3389/fphar.2019.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizzo G., De Santis M., Bosello S.L., Fedele A.L., Peluso G., Gremese E., Tolusso B., Ferraccioli G. Synovial fluid-derived T helper 17 cells correlate with inflammatory activity in arthritis, irrespectively of diagnosis. Clin. Immunol. 2011;138:107–116. doi: 10.1016/j.clim.2010.10.002. [DOI] [PubMed] [Google Scholar]