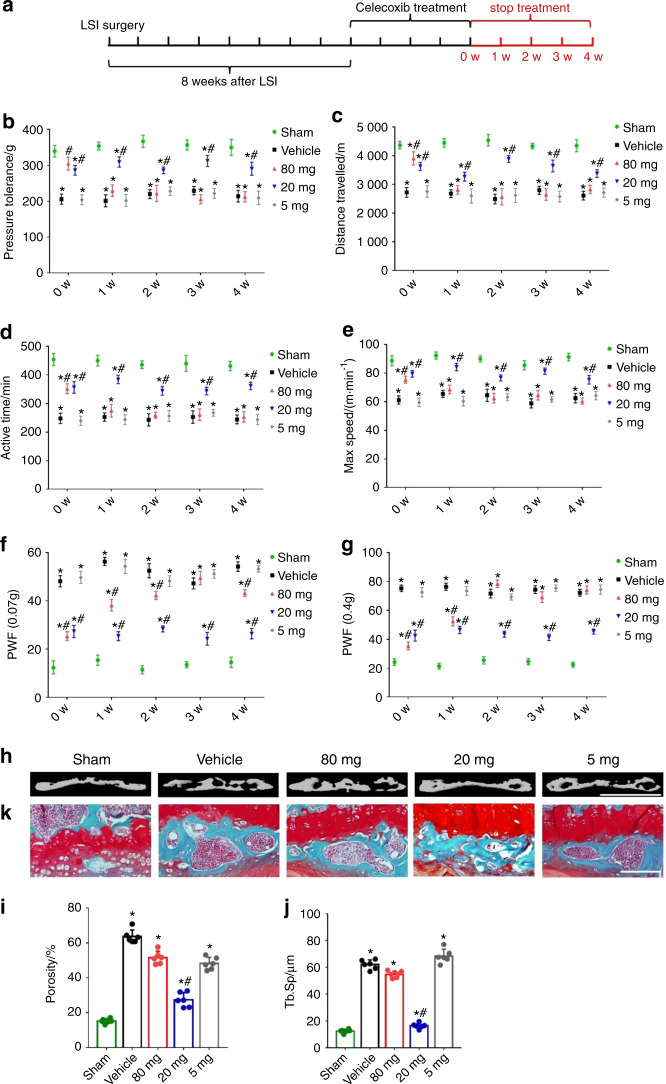
Fig. 7.
Spinal pain hypersensitivity remained relieved after discontinuation of low-dose celecoxib treatment. a Spinal pain and endplate porosity were measured at 4 weeks after discontinuation of celecoxib or vehicle treatment. b Vocalization thresholds of the mice with LSI surgery at 4 weeks after the discontinuation of celecoxib or vehicle treatment. Spontaneous activity results, including distance traveled (c), active time per 24 h (d), and maximum speed (e). f, g The PWF in the von Frey test (0.07 g and 0.4 g). h Representative μCT images of the L4-L5 caudal endplates at 4 weeks after the discontinuation of celecoxib or vehicle treatment. Scale bars, 1 mm. Quantitative analysis of the total porosity (i) and trabecular separation (j) of the L4-L5 caudal endplates based on the μCT images. *P < 0.05 compared with the sham group and #P < 0.05 compared with the vehicle group at the corresponding time points. n = 6 per group. k Representative safranin O and fast green staining images of the L4-L5 caudal endplate sections at 4 weeks after the discontinuation of celecoxib or vehicle treatment. Scale bars, 50 μm