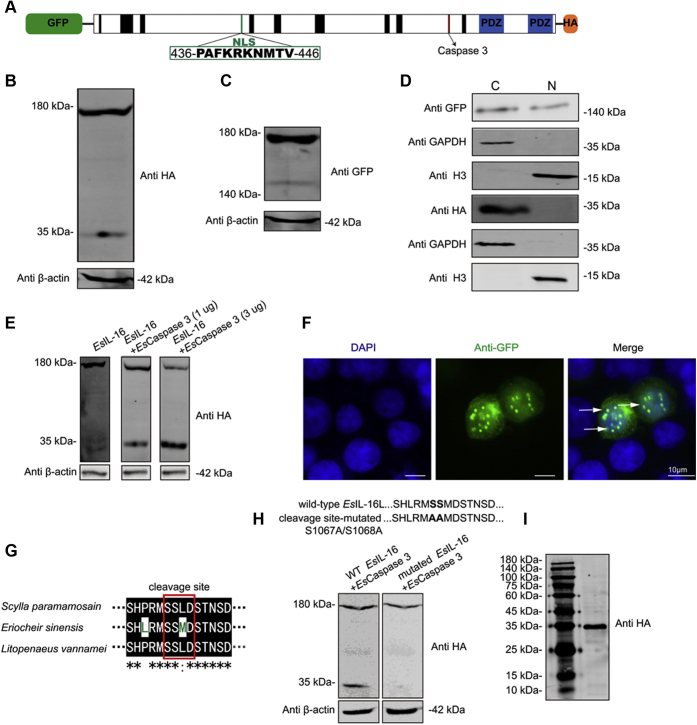
Figure 3.
Caspase-dependent pro-IL-16 cleavage and secretion.A, schematic representation of HEK293T cells expressed N-terminal GFP-tagged and C-terminal HA-tagged pro-EsIL-16 protein. B and C, Western blotting showing the cleavage products of the (B) C-terminal FLAG-tagged mature EsIL-16 and (C) the N-terminal GFP-tagged EsIL-16 prodomain. D, Western blotting showed the N-terminal GFP-tagged EsIL-16 translocated into nuclei, rather than C-terminal FLAG-tagged mature EsIL-16. E, Western blotting showed that Escaspase-3 is responsible for EsIL-16 cleavage. Cells that only transfected with EsIL-16 cannot generate a small fragment, cells that cotransfected with EsIL-16 and low concentration (1 μg) of Escaspase-3 generate clearly ~34 kDa small fragment, cells that cotransfected with EsIL-16 and high concentration (3 μg) of Escaspase-3 generate a strong ~34 kDa fragment. F, immunostaining shown nuclear imported N-terminal GFP-tagged EsIL-16. G, sequence alignment between caspase-3 cleavage site from three crustacean species showed comparative similar regions from Eriocheir sinensis S1062 to D1075. H, mutated E. sinensis caspase-3 cleavage site with indicated point mutants that displayed in bold and named wildtype EsIL-16 and cleavage site-mutated, respectively (upper panel). Western blot analysis showed after Escaspase-3 (1 μg) cotransfected with wildtype EsIL-16 or mutated EsIL-16, the wildtype EsIL-16 can generate a cleavage protein fragment with molecular weight of about ~34 kDa, but this fragment was absent in cleavage site–mutated EsIL-16 (latter panel). I, Western blotting showed that the C-terminal FLAG-tagged mature EsIL-16 is secreted into the extracellular region. Data are representative of three independent repeats. HA, hemagglutinin; HEK293T, human embryonic kidney 293T; IL-16, interleukin 16.