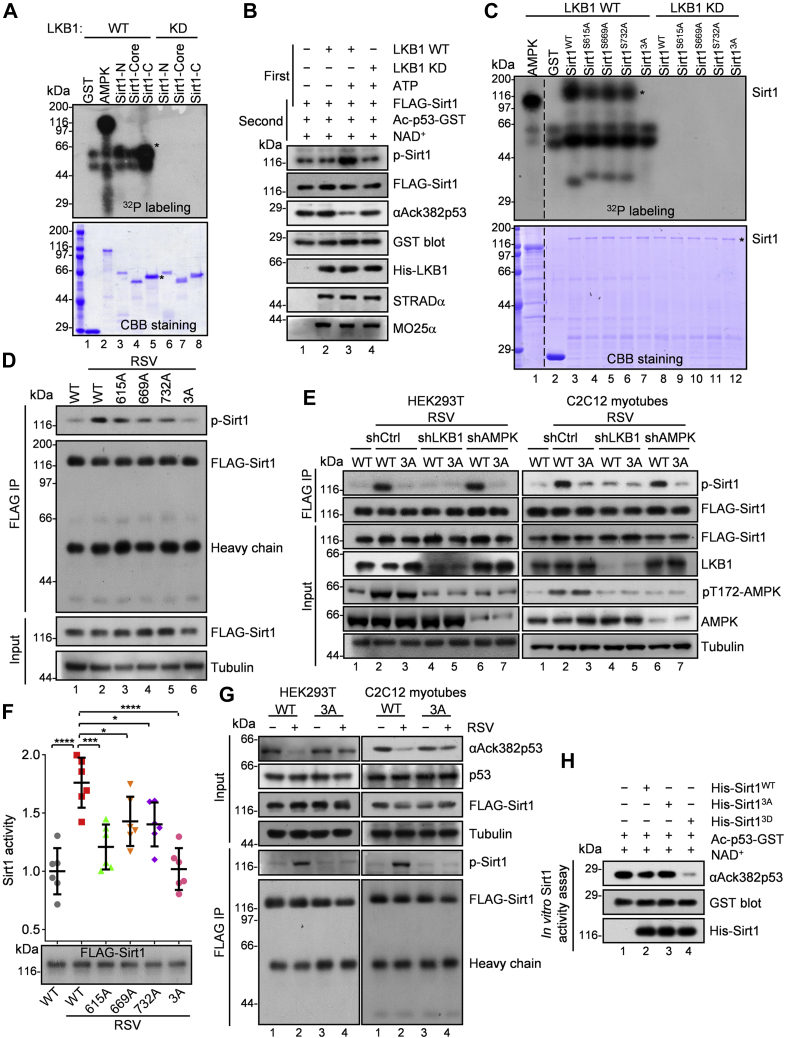
Figure 2.
LKB1 activates Sirt1 by direct phosphorylation.A, in vitro kinase assay using recombinant LKB1 kinase and purified GST-Sirt1 truncations. Samples were immunoblotted as indicated. MBP-AMPK incubated with LKB1 kinase was as positive control. Asterisks mark the C terminus of Sirt1. B, LKB1-dependent phosphorylation of Sirt1 is positively correlated with its deacetylase activity. HEK293T cells were infected with lentivirus-based particles expressing FLAG-Sirt1 and FLAG-Sirt1 was immunopurified by anti-FLAG. Precipitated FLAG-Sirt1 (2 μg), 100 ng recombinant LKB1 kinase (WT or kinase dead mutant), and 50 μM ATP were incubated in 40 μl kinase assay buffer at 30 °C for 30 min. FLAG-Sirt1 was spun down and 100 ng FLAG-Sirt1 was incubated with 1 mM NAD+ and 2 μg GST-tagged K382ac p53 peptide at 37 °C for 30 min in 40 μl Sirt1 assay buffer. The acetylation level of K382 site was analyzed by using anti-acetyl-p53 K382 antibody, and the phosphorylation level of Sirt1 was analyzed by using anti-phospho-serine/threonine. Representative of three independent experiments. p53 K382 is the deacetylation site of Sirt1 and ack382-p53 is the marker of Sirt1 activity (6, 86). C, in vitro kinase assay using recombinant LKB1 kinase and purified GST-Sirt1 WT or mutants. Samples were subjected to immunoblotting as indicated. Asterisks mark the proteins of interest. D, HEK293T cells stably expressing Sirt1 shRNA were transfected with lentivirus-based particles expressing FLAG-Sirt1 WT, FLAG-Sirt1 S615A, FLAG-Sirt1 S669A, FLAG-Sirt1 S732A, or FLAG-Sirt1 3A for 12 h. After 48 h, cells were treated with 25 μM resveratrol for 6 h. FLAG-Sirt1 WT or mutant proteins were immunoprecipitated by anti-FLAG. Phosphorylation level of Sirt1 was analyzed by using anti-phospho-serine/threonine. The 3A mutation, Ser615, Ser669, and Ser732 were replaced by Ala. E, phosphorylation of Sirt1 in resveratrol-treated LKB1-depleted cells expressing FLAG-Sirt1 WT or 3A mutant. Cells were infected with lentivirus-based particles expressing Sirt1 shRNA, and particles expressing shRNA control, AMPK shRNA, or LKB1 shRNA for 12 h, and 36 h later gene-depleted cells were infected with virus particles expressing FLAG-Sirt1 WT or 3A mutant for 12 h. After 36 h, cells were treated with 25 μM resveratrol for 6 h. FLAG-Sirt1 proteins were immunoprecipitated and immunoblotted with anti-phospho-serine/threonine. Representative of three independent experiments. The pT172-AMPK, marker of LKB1 activity. F, the activity of Sirt1 WT or mutants that were quantified by using a fluorophore-conjugated acetylated p53 peptide. HEK293T cells were lentivirus-based particles expressing Sirt1 shRNA for 12 h, and 36 h later gene-depleted cells were infected with virus particles expressing FLAG-Sirt1 WT or mutants for 12 h. After 48 h, cells were treated with 25 μM resveratrol for 6 h. FLAG-Sirt1 proteins were immunopurified by anti-FLAG and eluted by 3×FLAG peptide. And 50 ng eluted FLAG-Sirt1 were incubated with 1 mM of NAD+ and 200 μM fluorescently labeled acetylated p53 peptide in Sirt1 assay buffer at 37 °C for 30 min and the reaction was stopped with developer solution containing 2 mM nicotinamide. Sirt1 activity was assessed by measuring the fluorescent emission at 460 nm, following excitation at 360 nm. Data represents mean ± SD. The experiment was repeated six times independently. Statistical significance was determined by Dunnett's multiple comparisons test. ∗∗∗∗p (WT, WT RSV) < 0.0001. ∗∗∗p (WT, 615A) = 0.0002. ∗p (WT, 669A) = 0.0283. ∗p (WT, 732A) = 0.0164. ∗∗∗∗p (WT, 3A) < 0.0001. 615A, 669A, 732A, and 3A were replaced by Ala. G, LKB1-mediated phosphorylation of Sirt1 is positively correlated with its deacetylase activity in resveratrol-treated Sirt1-depleted cells expressing FLAG-Sirt1 WT or 3A mutant. Cells were infected with lentivirus-based particles expressing Sirt1 shRNA for 12 h, and 36 h later gene-depleted cells were infected with virus particles expressing FLAG-Sirt1 WT or 3A mutant for 12 h. After 36 h, cells were pretreated with 1 μM doxorubicin for 1 h to increase in vivo K382 acetylation of p53 (deacetylation site of Sirt1) and then were treated with 25 μM resveratrol for 6 h. The whole-cell lysate (WCL) was immunoblotted with anti-acetyl-p53 K382. FLAG-Sirt1 proteins were immunoprecipitated and immunoblotted with anti-phospho-serine/threonine. Representative of three independent experiments. The p53 K382 is the deacetylation site of Sirt1 and ack382-p53 is the marker of Sirt1 activity (6, 86). H, in vitro Sirt1 deacetylase activity assay. Baculovirus expressed His-tagged Sirt1 deacetylase (aa 193–747, Abcam, recombinant human Sirt1 protein, ab101130) WT, 3A mutant, or 3D mutant was incubated with 1 mM NAD+ and 2 μg GST-tagged K382ac p53 peptide from Escherichia coli at 37 °C for 30 min in 40 μl Sirt1 assay buffer. Acetylation level of K382 site was analyzed by using anti-acetyl-p53 K382 antibody. Representative of three independent experiments. The 3D mutation, Ser615, Ser669, and Ser732 were replaced by Asp. The p53 K382 is the deacetylation site of Sirt1 and ack382-p53 is the marker of Sirt1 activity (6, 86). 3A, Ser615, Ser669, and Ser732; 615A, Ser615Ala; 669A, Ser669Ala; 732A, Ser732Ala; KD, kinase dead; LKB1, Lys78Met; RSV, resveratrol; Sirt1-C, C terminus, aa 511 to 747; Sirt1-Core, core domain, aa 234 to 510; Sirt1-N, N terminus, aa 1 to 233.