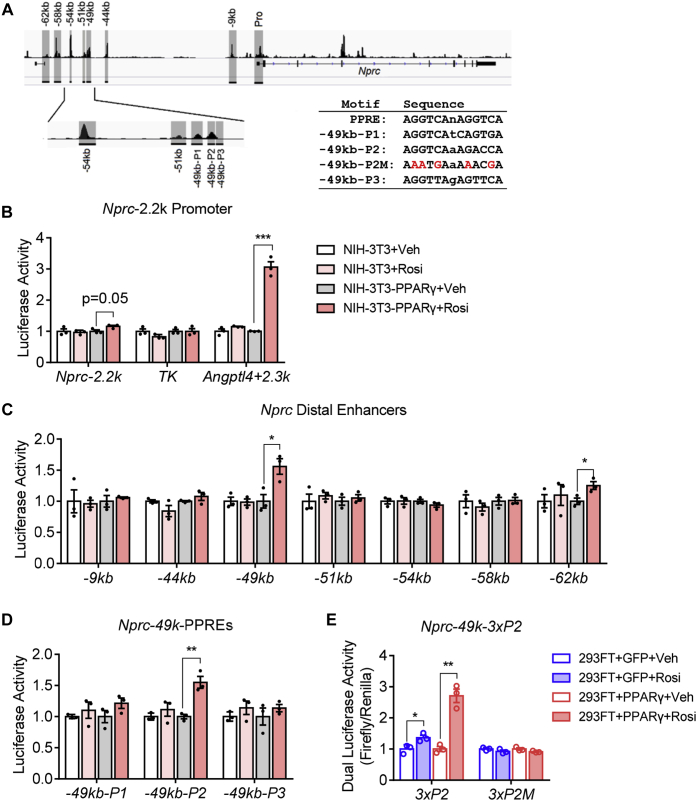
Figure 5.
Rosiglitazone increases Nprc distal enhancer activity but not proximal promoter activity alone.A, Nprc promoter (Pro), distal PPARγ enhancers (−9 kb, −44 kb, −49 kb, −51 kb, −54 kb, −58 kb, and 62 kb), and the three PPARγ response elements (−49 kb—P1, P2, and P3) are shown in gray and were cloned for luciferase reporter analysis. The sequences of the consensus PPRE motif and the Nprc PPREs are listed in inset. B, luciferase activity of Nprc promoter (−2233 bp to +1 bp, mNprc-2.2k). TK promoter alone (TK) and TK promoter with a PPARγ enhancer from the Angptl4 gene (Angptl4+2.3k) were used as the negative and positive controls, respectively. C, luciferase activity of the Nprc distal enhancer fragments (−9 kb, −44 kb, −49 kb, −51 kb, −54 kb, −58 kb, and −62 kb). D, enhancer activity of the Nprc distal PPREs in the −49 kb fragment (P1–P3). For reporter assays, NIH-3T3 and NIH-3T3 stably expressing PPARγ (NIH-3T3-PPARγ) cells were transfected with reporter plasmids and treated with vehicle (Veh) or 1 μM rosiglitazone (Rosi) for 48 h. Luciferase activity was normalized to protein concentrations. E, dual-luciferase assay of the −49kb-3xP2. 293FT cells were transfected with indicated reporter plasmids in combination with GFP or PPARγ and treated with vehicle (Veh) or 1 μM rosiglitazone (Rosi) for 24 h. Data were representative of at least three independent experiments. Student's t test, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. NIH, National Institutes of Health; Nprc, NP receptor C; PPARγ, peroxisome proliferator–activated receptor gamma; PPRE, PPARγ response element.