Abstract
Osteosarcoma (OS) is a malignant tumor prevalent in adolescents; however, a clinically effective treatment for this malignancy is lacking. The lack of effective treatment methods and factors, such as recurrence and drug resistance, further dampen the prospect of clinically treating OS. In recent years, small molecule microRNAs (miRNAs) with a length of approximately 20-24 nucleotides have gradually attracted the attention of the medical community. Studies have found that miRNAs can regulate the cell cycle, apoptosis, cell proliferation, and cell proliferation. The metabolic response of cancer cells, invasion and metastasis of cancer cells, and angiogenesis play an important role in the process of tumorigenesis. miRNAs regulate gene expression by regulating mRNA expression after transcription. A large amount of data from many studies indicate that they have diagnostic and prognostic biomarker effects in OS and are involved in regulating the metabolism of cancer cells and resistance or sensitivity to chemotherapy drugs. Chemotherapy resistance is one of the most critical problems in clinically treating OS. A large number of basic studies and systematic summaries are required to provide a theoretical basis for elucidating the mechanism and drug development of chemotherapeutic agents. Therefore, this article discusses the role of miRNAs in OS resistance. Herein, the related research progress of the studies is reviewed to provide more useful information for the development of effective therapy.
Keywords: microRNA, chemotherapeutic drugs, osteosarcoma, drug resistance, malignant, prognostic marker
Introduction
Osteosarcoma (OS) is a malignant bone tumor that originates from osteoblast mesenchymal cells. The most common site of OS is the metaphysis of the long bone, such as the distal femur and proximal tibia. OS has a high degree of malignancy and poor prognosis; therefore, its current treatment is mainly surgery combined with chemotherapy. 1 In addition, OS has a strong ability to invade and metastasize, such that 30% of patients still undergo lung and other organ metastases after undergoing surgical treatment, which is also one of the factors that lead to poor prognosis and the low survival rate of OS patients. 2 The onset of most OSs is hidden and difficult to detect in the early stage. Currently, neoadjuvant chemotherapy and adjuvant chemotherapy are very popular in the clinical treatment of OS. Therefore, at present, drug resistance in OS is a notable roadblock for the successful prognosis of OS. Currently, the main clinical chemotherapeutic drugs are platinum, doxorubicin, and methotrexate. Drug resistance is a common phenomenon in the process of chemotherapy and can be divided into intrinsic drug resistance and acquired drug resistance. However, the emergence and development of drug resistance seriously limit the therapeutic effect. As a result, the long-term improvement in the 5-year survival rate of OS patients is not obvious. Therefore, solving the problem of drug resistance is important for the treatment of OS.
MicroRNAs (miRNAs) are small molecule RNAs encoded by genes that can regulate the expression of target genes at the post-transcriptional or translational level. Studies have shown that there are a large number of differentially expressed miRNAs in OS tissues and cell lines. Some of these miRNAs with different expression levels are related to the occurrence and development of OS, and some can regulate the resistance of OS. Therefore, exploring the role and mechanism of miRNAs can provide new targets and ideas for the improvement and solution of drug resistance in OS.
Search Strategy
Related articles were searched in the Medline database with the English search term “MicroRNA, Chemotherapy Resistance, Osteosarcoma,” and a total of 120 related articles were retrieved.
Inclusion criteria: articles directly related to the study of miRNA in osteosarcoma resistance and corresponding previous basic research; similar research ideas selected the latest articles published in authoritative journals. Exclusion criteria: repeated or retrospective research. By reading the title and abstract, articles that are not related to the subject of the review and highly similar research are excluded. Finally, the reference list contains 46 articles.
Regulatory Effect of miRNA on Cisplatin Resistance in OS
Cisplatin (CDDP) is a widely used antineoplastic drug in clinical settings. OS is often treated using CDDP combined with other drugs. In this process, the decrease in tumor histochemical sensitivity is an important reason for the failure of CDDP chemotherapy. Some miRNAs play an important regulatory role in the process of OS drug resistance. Therefore, it is of great clinical value to clarify the regulatory mechanism of miRNAs in OS CDDP drug resistance, which is expected to provide new ideas for solving the problem of OS CDDP drug resistance.
A large number of studies have shown that miRNAs can directly or indirectly target the mRNA of the target gene and regulate the drug resistance of CDDP. Liang et al 3 found that Apurinic and pyrimidine endonuclease (APEI) is usually highly expressed in OS, and is positively correlated with angiogenesis in OS. MiRNA-765 can target and inhibit the expression of APEI, weaken the damage repairability of DNA, and improve the sensitivity to OS. At the same time, the combination of CDDP and miRNA-765 can inhibit the expression of related angiogenic factors, such as FGF2, TGF-β, and MVD, and improve the sensitivity of the treatment. In a mouse model, positive miR-765 significantly inhibited tumor growth. Song et al 4 found that the expression of miR-340-5p was low in anti-CDDP MG-63 and Saos-2 cell lines, while the forced expression of miRNA-340-5p decreased the expression of the target gene, LPAAT β. It not only reduced the half-lethal dose (IC50) of CDDP but also reduced the chemical resistance to CDDP. Zhou et al 5 found that compared to normal osteoblasts, miRNA-22 expression was lower and S100 calcium-binding protein A11 (S100A11) was negatively regulated in the OS tissues and MG-63 cells. Overexpression of miRNA-22 inhibited the proliferation and migration of MG-63 cells and increased the sensitivity of CDDP therapy. MiRNA-377 often enhances the sensitivity of OS during chemotherapy. 6 After reversing the low expression of miRNA-377 in OS, the chemically-resistant OS and CDDP-resistant cell lines were re-sensitized to CDDP and restored Caspase-3-dependent apoptosis induced by CDDP. MiRNA-377 can also directly bind to the 3’ UTR of XIAP to inhibit its expression, which indicates that the miRNA-377/XIAP axis plays an important role in regulating drug resistance.
Studies by Yu et al 7 showed that the expression of miRNA-221 was upregulated in the OS cell lines, MG-63 and U2OS, while the transfection of miRNA-221 mimics resulted in higher resistance to CDDP in OS cell lines. At the same time, it could target and inhibit PPP2R2A. In contrast, restoring PP2R2AR in miRNA-221 cells can reverse the CDDP drug resistance.
MiRNA-138 is a tumor suppressor that is expressed at low levels in OS cell lines. 8 It can directly bind to the 3` UTR of EZH2. When the OS cell line was transfected with the mi-RNA138 mimic, the expression of EZH2 was inhibited, and the sensitivity of CDDP was enhanced.
Chen et al 9 found that the expression of miRNA-504 was increased in OS and MG-63 cells, which led to the inhibition of CDDP-induced apoptosis and cell cycle arrest, mainly due to the direct targeting and inhibition of p53 by miRNA-504. In contrast, inhibiting the expression of miRNA-504 could alleviate the drug resistance of CDDP. Jacques et al 10 found that a high expression of miRNA-193a-5p can induce high drug resistance in CDDP, and the target of its negative regulation is Tap73 β. After inhibiting the expression of miRNA-193a-5p, it can restore the level of Tap73 β in cells, thereby promoting the expression of Caspase-3/7, promoting the apoptosis of drug-resistant cells, and enhancing the sensitivity of OS cells to CDDP. Zhou et al 11 found that miRNA-33a is upregulated and negatively regulates the target gene, TWIST, in drug-resistant MG-63 and Saos-2 cells. When the expression of miRNA-33a was inhibited, the expression of TWIST was upregulated, which led to an increase in apoptosis induced by CDDP and reversal of drug resistance. Wang et al 12 followed up some patients with OS and found that the expression of miRNA-491 in their serum was downregulated. At the same time, in vitro and in vivo studies revealed that the overexpression of miRNA-491 inhibited lung metastasis of OS cells and enhanced tumor growth inhibition and apoptosis induced by CDDP. The molecular mechanism of this biological effect is achieved by directly targeting αB-crystallin (CRYAB). NEAT1 is a non-coding RNA (lncRNA) that acts as an oncogene. NEAT1 is overexpressed in OS tissues and MG-63 and HOS cell lines, which can negatively regulate the expression of miRNA-34c. When the level of miRNA-34c was decreased, the expression of Bcl-2 and CCND1 increased, which reduced the sensitivity of OS to CDDP and inhibited apoptosis and cell cycle arrest. In contrast, inhibiting the expression of NEAT could reverse this phenomenon. 13
MiRNA-133b is another miRNA that has potential as a biomarker in the treatment of OS. 14 Its expression in CDDP-resistant cells was higher than that in MG-63 cells. When its expression is inhibited, miRNA-133b can inhibit the invasion and metastasis of OS cells, weaken drug resistance, and enhance the chemotherapeutic effect of CDDP. Similarly, miRNA-21 has been shown to play a regulatory role in drug resistance. 15 When the miRNA-21 simulator was applied, the content of Bcl-2 increased almost synchronously, which weakened the cytotoxicity of CDDP but significantly improved after the inhibition of miRNA-21. In addition, the study found that quercetin, the active component of traditional Chinese medicine, can regulate the sensitivity of miR-217/KARS by adjusting the CDDP axis. 16
Similarly, the regulatory effect of miRNAs on some signaling pathways is one of the mechanisms of drug resistance of CDDP in OS (Table 1). Zhou et al 11 found that the negative regulation of miRNA-33a can directly target TWIST, while TWIST can downregulate ET-1/ETAR signaling by inhibiting the PI3K/Akt pathway, thereby making OS cells sensitive to CDDP. At the same time, miRNA-221 has also been proven to possess this function. 17 MiRNA-221 was highly expressed in OS and paratumoral tissues, while expression of PTEN, the target of negative regulation, was low. PTEN can regulate the drug resistance of OS cells to CDDP by regulating the PI3K/Akt pathway. Therefore, the miRNA-221/PTEN/PI3K/Akt signaling pathway may be an important part of the OS drug resistance regulation signal network. Liu et al 18 found that the expression of miRNA-100 is low in OS cell lines, but when miRNA-100 mimics are applied, it can directly inhibit the expression of the target gene, IGFIR, inhibit the PI3K/AKT and MAPK/ERK signaling pathways, promote apoptosis, and weaken the drug resistance of OS. Similarly, miRNA-34a has been shown to regulate signaling pathways. 19 When miRNA-34a is overexpressed and combined with CDDP, the c-Myc/Bim pathway can be activated to make OS cells sensitive to CDDP and induce apoptosis. Tang et al 20 found that miRNA-223 is a type of miRNA with low expression in OS, which can directly target and negatively regulate the expression of Hsp70; when miRNA-223 is overexpressed, it can inhibit the expression of Hsp70, thereby regulating the increase of JNK/JUN expression, which makes cells sensitive to CDDP. JUN can also bind to the promoter region of miRNA-223 to form a feedback loop and induce an increase in its expression. In addition, autophagy regulated by miRNAs can affect the resistance of OS cells to CDDP (Table 2). Li et al 21 found that after MG-63 cells were treated with CDDP to form an OS-resistant cell model, the expression of miRNA-199a-5p decreased, the negative regulatory target gene, Beclin1, expression and the ratio of LC3-II to LC3-I increased, indicating that autophagy was activated; forced overexpression of miRNA-199a-5p resulted in the opposite result, which not only inhibited autophagy but also enhanced the cytotoxicity of CDDP, and reversed the CDDP resistance of OS cells. MiRNA-22 also has similar functions. 22 The expression of miRNA-22 is low in OS cells. When miRNA-22 mimics are added to MG-63, targeting inhibits MTDH and reduces the expression of LC3, ATG5, and Beclin1, which inhibits autophagy and promotes chemosensitivity.
Table 1.
The Role of miRNA With Signal Pathway Regulation in the CDDP Resistance of OS.
| miRNA | Expression level in OS | Gene target | Signal pathway | Effect after intervention | References |
|---|---|---|---|---|---|
| miRNA-33a | ↑ | TWIST | PI3K/Akt, ET-1/ETAR | Increased chemotherapy sensitivity of CDDP | 11 |
| miRNA-221 | ↑ | PTEN | PI3K/Akt | Reduced CDDP resistance | 17 |
| miRNA-100 | ↓ | IGFIR | PI3K/Akt, MAPK/Erk | Reduced CDDP resistance | 18 |
| miRNA-34a | ↓ | – | c-Myc/Bim | Increased chemotherapy sensitivity of CDDP | 19 |
| miRNA-223 | ↓ | Hsp70 | JNK/JUN | Increased chemotherapy sensitivity of CDDP | 20 |
| miRNA-22 | ↓ | MTDH | LC3/ATG5/Beclin1 | Increased chemotherapy sensitivity of CDDP | 22 |
Table 2.
MiRNAs That Regulate Cell Autophagy in the Resistance of Osteosarcoma.
| miRNA | Target gene | OS cell types | Chemotherapy drugs | References |
|---|---|---|---|---|
| miRNA-199a-5p | Beclin1, LC3-II, LC3-I | MG63 | CDDP | 21 |
| miRNA-22 | MTDH, LC3, ATG5, beclin1 | MG63 | 22 | |
| miRNA-101 | AVOS, A2g4 | U-2 OS | Dox | 28 |
| miRNA-30a | Beclin-1, LC3-I, LC3-II | MG63 | 29 | |
| miRNA-590 | WIP1, p65 | Saos2, HOS, U-2 OS, MG63 | 30 | |
| miRNA-24 | Bim, Smac, DIABLO, XIAP, Caspase-3/7/9 | MG-63, HOS | 25 | |
| miRNA-140-5p | HMGN5 | MG-63, U-2 OS | MTX | 31 |
| miRNA-199a-3p | AK4, NF-κB | G-292, U-2 OS, HOS | CDDP, carb, dox | 32 |
| miRNA-140-5p | IP3K2 | Saos-2, MG-63 | Dox, CDDP | 33 |
Different miRNAs exhibit different expression changes in the osteosarcoma tissues. It is believed that after interfering with their expression levels to reverse these expression changes, different miRNAs can enhance the chemotherapeutic sensitivity of OS cells to CDDP or inhibit the resistance of OS cells to CDDP by targeting different molecular targets and regulating the activation of downstream signaling pathways.
The Role of miRNA in Adriamycin Resistance
Another common clinical chemotherapeutic drug, doxorubicin (Dox), is associated with the emergence and development of drug resistance. A large number of studies have shown that miRNAs play an important role in regulating Dox resistance in the treatment of OS. Lin et al 23 found that OS cell lines treated with a certain dose of Dox had time-dependent changes in miRNA-184, which peaked at 12 h and then decreased. MiRNA-184 can directly target Bcl2L1 and has a positive correlation with it. The use of miRNA-184 antagonists can promote the apoptosis of OS cells, while overexpression can inhibit apoptosis and promote the chemical resistance of OS to Dox. Sun et al 24 showed that miRNA-137-3p can regulate drug resistance in OS cells by inhibiting the expression of multiple growth factors. In OS-resistant cells, the expression of miRNA-137-3p was lower than that in normal OS cells, but overexpression of miRNA-137-3p downregulated the level of target gene, PTN, inhibited the proliferation of OS cells, and reversed drug resistance. In contrast, overexpression of PTN also weakened the inhibitory effect of miRNA-137-3p on the proliferation of drug-resistant OS cells. MiRNA-149 is a cancer-promoting factor, 25 and its expression is significantly increased in the OS tissues. It is negatively correlated with the target gene bone morphogenetic protein 9 (BMP9) and affects the expression of Caspase3 and PARP. When miRNA-149 was overexpressed in MG-63 cells, the sensitivity of Dox was weakened by the decrease in BMP9 expression. Zhou et al 26 found that miRNA-320 could regulate the drug resistance of OS. It can directly target SNHG12 and MCL-1 and downregulate their expression to reduce the half-lethal dose of Dox in the MG-63 cell line. Zhang et al 27 found that miRNA-301a directly negatively regulates AMPKα1. The experimental results showed that the drug-resistant OS cells treated with Dox induced the expression of miRNA-301a in a time-dependent manner and increased the expression of HMGCR. Upregulation of miRNA-301a can inhibit the expression of AMPKα1, which enhances the chemical resistance of OS cells to Dox.
Autophagy is an important factor affecting the drug resistance of OS (Table 2). Chang et al 28 found that miRNA-101 can block autophagy in OS and improve the chemosensitivity of Dox in vitro. The results also revealed that autophagy could be induced by a certain dose of Dox in U2-OS cells, while the expression of AVOS and another autophagy-related protein, A2g4, could be decreased after transfection of miRNA-101, which could block autophagy; this block could increase the sensitivity of OS cells to Dox. MiRNA-30a is another regulatory factor that affects drug resistance by affecting autophagy. 29 After treatment, the content of miRNA-30a decreased significantly, the expression of the target gene, Beclin1, increased, and the transformation of microtubule-related protein LC3-I to LC3-II also increased, suggesting that autophagy activity increased. Overexpression of miRNA-30a inhibits autophagy and promotes chemotherapy-induced apoptosis. In terms of signaling pathways, miRNA-590 plays a regulatory role. 30 Overexpression of miRNA-590 in OS cell lines can negatively regulate WIP1 to enhance the cytotoxicity of Dox and promote apoptosis. The main reason for this is that p65 can bind to the miRNA-590 promoter, thereby inhibiting the expression of miRNA-590. Sun et al 24 and other researchers have shown that miRNA-24 is highly expressed in OS cell lines. Inhibition of miRNA-24 and treatment with Dox significantly promoted apoptosis and alleviated drug resistance. The main mechanism is that the inhibition of miRNA-24 can upregulate the expression of its target gene, Bim, which can lead to the release of Smac/DIABLO from the mitochondria and bind to XIAP. This not only induces mitochondrial dysfunction but also triggers the activation of Caspase-3/7/9 and promotes apoptosis. To solve the problem of Dox drug resistance, summaries and further explorations can be carried out according to the above mechanisms or rules, to provide new targets and new ideas for clinical treatment.
Autophagy is one of the most important biological activities of tumor cells and activating the autophagy of tumor cells is one of the most important treatment strategies for malignant tumors. Some miRNAs regulate cytotoxic activity; therefore, these miRNAs can improve the chemotherapy sensitivity in OS drug-resistant cell lines.
Effect of miRNA on Methotrexate Resistance
Methotrexate (MTX) is an anti-metabolic drug that is used as an anti-tumor therapy. MTX is often used in combination with cisplatin and other drugs for the treatment of OS. However, there is a certain phenomenon of drug resistance to MTX, whether used alone or in combination. Many studies have shown that miRNAs also affect the chemosensitivity of MTX to some extent. The MiRNA-29 family is a type of tumor suppressor. 34 In drug-resistant cell lines treated with MTX and OS tissues with poor effects on chemotherapy, miRNA-29a/b/c was downregulated to varying degrees. By detection, the miRNA-29 family negatively regulates the expression of COL3A1 and MCL1. Overexpression of miRNA-29a/b/c can inhibit the expression of target genes, making MTX-resistant cells sensitive to chemotherapy and significantly promoting apoptosis.
Xu et al 35 found that miRNA-146-5p upregulates OS before and after chemotherapy and negatively regulates zinc finger protein 3 (ZNRF3) to promote the chemical resistance of OS cells to MTX, and this increased resistance is mediated by the Wnt/β-catenin signaling pathway. MiRNA-382 36 is another tumor suppressor factor downregulated in OS, which directly targets and negatively regulates KLF12 and HIPK3. Overexpression of miRNA-382 enhanced the sensitivity of OS cells to MTX-induced apoptosis. In fact, Meng et al 31 found that the expression of miRNA-140-5p in OS decreased, while overexpression of miRNA-140-5p reversed drug resistance. The main reason is that miRNA-140-5p targets HMGN5 and reduces its expression, thereby blocking autophagy and achieving chemical sensitization (Table 2).
The expression of miRNA-34c 37 is lower in OS, which is not sensitive to chemotherapy; however, when miRNA-34c is overexpressed, it can increase the level of Caspase-3 and promote apoptosis by targeting the inhibition of Notch1 and LEF1, thereby alleviating the resistance of OS to MTX.
The Role of miRNA in Multi-Drug Resistance in OS
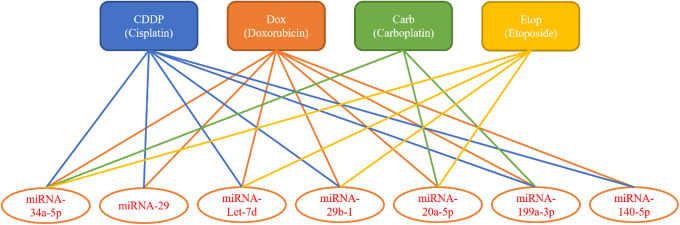
In intrinsic drug resistance at the beginning of chemotherapy and acquired drug resistance in the later stages, tumor cells may produce resistance to a variety of drugs. As a result, the effect of chemotherapy is limited and negatively affects the prognosis of the disease. Therefore, exploring and understanding the mechanism and principle of miRNA in multidrug resistance in OS can provide a new target for solving the phenomenon of drug resistance (Figure 1).
Figure 1.
MiRNAs related to multidrug resistance in OS.
Because the clinical treatment of OS often uses a combination of multiple chemotherapeutic drugs, the occurrence of multidrug resistance in OS seriously affects the chemotherapeutic effect on OS. Therefore, the discovery of miRNAs related to multidrug resistance in OS can provide a solution to this problem. Therefore, we used Figure 1 to describe the correspondence between chemotherapeutic drugs and lncRNAs to provide systematic theoretical evidence for the solution of the problem of multidrug resistance in OS.
Pu et al 38 showed that miRNA-34a-5p can promote multidrug resistance in OS cells. In this study, AGTR1 is a direct target of mir-34a-5p and negatively regulates the multidrug resistance of OS [DOX, CDDP, etoposide (Etop), and carboplatin (Carb)]. MiRNA-34a-5p can also promote poly chemical resistance of OS by directly downregulating the DLL1 gene, in which the ATF2/ATF3/ATF4 signaling pathway may be involved in this promoting effect. Osaki et al 39 found that the upregulation of miRNA-29 in OS can inhibit the expression of the anti-apoptosis factor, MCL1, thereby increasing the sensitivity of OS to CDDP and Dox. In the aspect of OS stem cells, Fiore et al 40,41 found that let-7d was downregulated in the 3AB-OS cancer stem cell (CSC) cell line derived from MG63. Overexpression of let-7d leads to the chemical resistance of cell lines to a variety of chemotherapeutic drugs, accompanied by the inhibition of Caspase-3 and an increase in Bcl-2 expression. In contrast, overexpression of miRNA-29b-1 in this cell line can reverse drug resistance and increase the apoptotic rate induced by many chemotherapeutic drugs. MiRNA-20a-5p is another adjustable multidrug resistance miRNA. 42 Compared with the drug-resistant (SJSA) cell line, the expression of miRNA-20a-5p was significantly higher in OS-sensitive (GMEC 292) cell lines, while the forced expression of miRNA-20a-5p in SJSA cells counteracted the drug resistance of OS. The main mechanism is to inhibit the expression of the target gene, SDC2, and promote apoptosis induced by chemotherapeutic drugs, such as Dox, Etop, and Carb. In the aspect of autophagy, miRNA-199a-3p can promote multidrug resistance by inhibiting the expression of the target gene, AK4. 43 In this process, reducing miRNA-199a-3p can upregulate the activated NF-κB pathway. Concerning autophagy, Wei et al 32 found that miRNA-140-5p was upregulated in OS cells treated with CDDP and Dox, which inhibited the IP3K2 gene and upregulated autophagy, resulting in drug resistance of OS. In addition, miRNA-140-5p can upregulate autophagy by inhibiting the IP3K2 gene and producing chemical resistance to CDDP and Dox (Table 2). 33
Summary and Prospect
Since miRNA was discovered by Lee 33 in the study of developmental defects in Caenorhabditis elegans in 1993, related research on miRNA has developed rapidly and has results in remarkable amounts of research data, including the regulatory role of miRNAs in drug resistance in OS. It involves the regulation of miRNA in the cell cycle, cell proliferation, apoptosis, and autophagy in OS and its cell lines. However, most of these studies focused only on a single target and were scattered. Therefore, we believe that future research on miRNAs in OS resistance should focus more on the regulation of specific upstream and downstream molecules of miRNAs and their signaling pathways. The interaction mechanism between different miRNAs is also a key and difficult problem. If we can clarify the above 2 questions and build a complete miRNA regulatory network on this basis, we will be able to comprehensively and thoroughly clarify the regulatory mechanism of miRNAs in the drug resistance of osteosarcoma and provide a theoretical basis for the development of gene therapy targets. Once this regulatory network is established, researchers can find one or more important “central targets” through the network to optimize the inhibition of OS resistance by blocking or activating one or more molecules.
Currently, the use of miRNA drugs in the treatment of hepatitis C has been carried out in phase II clinical trials, 44 which play a significant role in encouraging miRNA-related drugs to improve chemotherapy resistance. In a large number of experiments, various miRNA mimics, inhibitors, and agonists have been widely used, and some drugs such as quercetin and lncRNA have been shown to regulate miRNA. Therefore, future research should focus on the exploration of various microRNA-related drugs and the development of a combination of different drugs in the treatment of OS to provide a reliable and effective solution for the clinical treatment of OS.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Abbreviations
- APEI
Apurinic and pyrimidine endonuclease
- BMP9
bone morphogenetic protein 9
- CDDP
Cisplatin
- EZH2
Enhancer of zeste homolog 2
- FGF2
fibroblast growth factor 2
- LPAAT-β
Lysophosphatidic acid acyltransferase beta
- miRNAs
microRNAs
- MVD
Mevalonate diphosphate decarboxylase
- OS
osteosarcoma
- XIAP
X-linked inhibitor of apoptosis
Authors’ Note: Zhaopeng Tang and Yubao Lu contributed equally to this study and share first authorship. This article is a review article and does not involve ethical issues.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the Cuiying Scientific Training Program for Undergraduates of Lanzhou University Second Hospital (CYXZ2020-03).
ORCID iD: Yubao Lu, PhD  https://orcid.org/0000-0003-4375-8316
https://orcid.org/0000-0003-4375-8316
References
- 1. Smolle MA, Pichler M. The role of long non-coding RNAs in osteosarcoma. Noncoding RNA. 2018;4(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xiao X, Wang W, Wang Z. The role of chemotherapy for metastatic, relapsed and refractory osteosarcoma. J Pediatric Drugs. 2014;16(6):503–512. [DOI] [PubMed] [Google Scholar]
- 3. Liang W, Li C, Li M, Wang D, Zhong Z. MicroRNA-765 sensitizes osteosarcoma cells to cisplatin via downregulating APE1 expression. Onco Targets Ther. 2019;12:7203–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Song L, Duan P, Gan Y, et al. MicroRNA-340-5p modulates cisplatin resistance by targeting LPAATβ in osteosarcoma. Braz J Med Biol Res. 2017;50(5):e6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou X, Natino D, Zhai X, Gao Z, He X. MicroRNA-22 inhibits the proliferation and migration, and increases the cisplatin sensitivity, of osteosarcoma cells. Mol Med Rep. 2018;17(5):7209–7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu XG, Xu J, Li F, Li MJ, Hu T. Down-regulation of miR-377 contributes to cisplatin resistance by targeting XIAP in osteosarcoma. Eur Rev Med Pharmacol Sci. 2018;22(5):1249–1257. [DOI] [PubMed] [Google Scholar]
- 7. Yu WC, Chen HH, Qu YY, Xu CW, Yang C, Liu Y. MicroRNA-221 promotes cisplatin resistance in osteosarcoma cells by targeting PPP2R2A. Biosci Rep. 2019;39(7):BSR20190198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu Z, Tang J, Wang J, Duan G, Zhou L, Zhou X. MiR-138 acts as a tumor suppressor by targeting EZH2 and enhances cisplatin-induced apoptosis in osteosarcoma cells. PLoS One. 2016;11(3):e0150026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen X, Lv C, Zhu X, et al. MicroRNA-504 modulates osteosarcoma cell chemoresistance to cisplatin by targeting p53. Oncol Lett. 2019;17(2):1664–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacques C, Calleja LR, Baud’huin M, et al. miRNA-193a-5p repression of p73 controls Cisplatin chemoresistance in primary bone tumors. Oncotarget. 2016;7(34):54503–54514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou Y, Huang Z, Wu S, Zang X, Liu M, Shi J. miR-33a is up-regulated in chemoresistant osteosarcoma and promotes osteosarcoma cell resistance to cisplatin by down-regulating TWIST. J Exp Clin Cancer Res. 2014;33(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang SN, Luo S, Liu C, et al. miR-491 inhibits osteosarcoma lung metastasis and chemoresistance by targeting αB-crystallin. Mol Ther. 2017;25(9):2140–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu Y, Yang Q, Wang L, et al. Knockdown of the oncogene lncRNA NEAT1 restores the availability of miR-34c and improves the sensitivity to cisplatin in osteosarcoma. Biosci Rep. 2018;38(3):BSR20180375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zou Y, Yang J, Wu J, Luo C, Huang Y. miR-133b induces chemoresistance of osteosarcoma cells to cisplatin treatment by promoting cell death, migration and invasion. Oncol Lett. 2018;15(1):1097–1102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Ziyan W, Yang L. MicroRNA-21 regulates the sensitivity to cisplatin in a human osteosarcoma cell line. Ir J Med Sci. 2016;185(1):85–91. [DOI] [PubMed] [Google Scholar]
- 16. Zhang X, Guo Q, Chen J, Chen Z. Quercetin enhances cisplatin sensitivity of human osteosarcoma cells by modulating microRNA-217-KRAS axis. Mol Cells. 2015;38(7):638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao G, Cai C, Yang T, et al. MicroRNA-221 induces cell survival and cisplatin resistance through PI3K/Akt pathway in human osteosarcoma. PLoS One. 2013;8(1):e53906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Y, Zhu ST, Wang X, et al. MiR-100 inhibits osteosarcoma cell proliferation, migration, and invasion and enhances chemosensitivity by targeting IGFIR. Technol Cancer Res Treat. 2016;15(5):NP40–NP48. [DOI] [PubMed] [Google Scholar]
- 19. Li QC, Xu H, Wang X, Wang T, Wu J. miR-34a increases cisplatin sensitivity of osteosarcoma cells in vitro through up-regulation of c-Myc and Bim signal. Cancer Biomark. 2017;21(1):135–144. [DOI] [PubMed] [Google Scholar]
- 20. Tang Q, Yuan Q, Li H, et al. miR-223/Hsp70/JNK/JUN/miR-223 feedback loop modulates the chemoresistance of osteosarcoma to cisplatin. Biochem Biophys Res Commun. 2018;497(3):827–834. [DOI] [PubMed] [Google Scholar]
- 21. Li Y, Jiang W, Hu Y, et al. MicroRNA-199a-5p inhibits cisplatin-induced drug resistance via inhibition of autophagy in osteosarcoma cells. Oncol Lett. 2016;12(5):4203–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang P, Zhao ZQ, Guo SB, et al. Roles of microRNA-22 in suppressing proliferation and promoting sensitivity of osteosarcoma cells via metadherin-mediated autophagy. Orthop Surg. 2019;11(2):285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin BC, Huang D, Yu CQ, et al. MicroRNA-184 modulates doxorubicin resistance in osteosarcoma cells by targeting BCL2L1. Med Sci Monit. 2016;22:1761–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun Y, He N, Dong Y, Jiang C. MiR-24-BIM-Smac/DIABLO axis controls the sensitivity to doxorubicin treatment in osteosarcoma. Sci Rep. 2016;6(1):34238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xie Z, Xu J, Peng L, Gao Y, Zhao H, Qu Y. miR-149 promotes human osteocarcinoma progression via targeting Bone Morphogenetic Protein 9 (BMP9). Biotechnol Lett. 2018;40(1):47–55. [DOI] [PubMed] [Google Scholar]
- 26. Zhou B, Li L, Li Y, Sun H, Zeng C. Long noncoding RNA SNHG12 mediates doxorubicin resistance of osteosarcoma via miR-320a/MCL1 axis. Biomed Pharmacother. 2018;106:850–857. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y, Duan G, Feng S. MicroRNA-301a modulates doxorubicin resistance in osteosarcoma cells by targeting AMP-activated protein kinase alpha 1. Biochem Biophys Res Commun. 2015;459(3):367–373. [DOI] [PubMed] [Google Scholar]
- 28. Chang Z, Huo L, Li K, Wu Y, Hu Z. Blocked autophagy by miR-101 enhances osteosarcoma cell chemosensitivity in vitro. Sci World J. 2014;2014:794756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu R, Liu S, Chen H, Lao L. MicroRNA-30a downregulation contributes to chemoresistance of osteosarcoma cells through activating beclin-1-mediated autophagy. Oncol Rep. 2016;35(3):1757–1763. [DOI] [PubMed] [Google Scholar]
- 30. Long X, Lin XJ. P65-mediated miR-590 inhibition modulates the chemoresistance of osteosarcoma to doxorubicin through targeting wild-type p53-induced phosphatase 1. J Cell Biochem. 2019;120(4):5652–5665. [DOI] [PubMed] [Google Scholar]
- 31. Meng Y, Gao R, Ma J, et al. MicroRNA-140-5p regulates osteosarcoma chemoresistance by targeting HMGN5 and autophagy. Sci Rep. 2017;7(1):416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei R, Cao G, Deng Z, Su J, Cai L. miR-140-5p attenuates chemotherapeutic drug-induced cell death by regulating autophagy through inositol 1,4,5-trisphosphate kinase 2 (IP3k2) in human osteosarcoma cells. Biosci Rep. 2016;36(5):e00392–e00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. [DOI] [PubMed] [Google Scholar]
- 34. Xu W, Li Z, Zhu X, Xu R, Xu Y. miR-29 family inhibits resistance to methotrexate and promotes cell apoptosis by targeting COL3A1 and MCL1 in osteosarcoma. Med Sci Monit. 2018;24:8812–8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu E, Zhao J, Ma J, et al. miR-146b-5p promotes invasion and metastasis contributing to chemoresistance in osteosarcoma by targeting zinc and ring finger 3. Oncol Rep. 2016;35(1):275–283. [DOI] [PubMed] [Google Scholar]
- 36. Xu M, Jin H, Xu CX, et al. miR-382 inhibits tumor growth and enhance chemosensitivity in osteosarcoma. Oncotarget. 2014;5(19):9472–9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu M, Jin H, Xu CX, Bi WZ, Wang Y. MiR-34c inhibits osteosarcoma metastasis and chemoresistance. Med Oncol. 2014;31(6):972. [DOI] [PubMed] [Google Scholar]
- 38. Pu Y, Zhao F, Li Y, et al. The miR-34a-5p promotes the multi-chemoresistance of osteosarcoma via repression of the AGTR1 gen. BMC Cancer. 2017;17(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Osaki S, Tazawa H, Hasei J, et al. Ablation of MCL1 expression by virally induced microRNA-29 reverses chemoresistance in human osteosarcomas. Sci Rep. 2016;6(1):28953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fiore RD, Drago-Ferrante R, Pentimalli F, et al. Let-7d miRNA shows both antioncogenic and oncogenic functions in osteosarcoma-derived 3AB-OS cancer stem cells. J Cell Physiol. 2016;231(8):1832–1841. [DOI] [PubMed] [Google Scholar]
- 41. Fiore RD, Drago-Ferrante R, Pentimalli F, et al. MicroRNA-29b-1 impairs in vitro cell proliferation, self-renewal and chemoresistance of human osteosarcoma 3AB-OS cancer stem cells. Int J Oncol. 2014;45(5):2013–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao F, Pu Y, Cui M, Wang H, Cai S. MiR-20a-5p represses the multi-drug resistance of osteosarcoma by targeting the SDC2 gene. Cancer Cell Int. 2017;17(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lei W, Yan C, Ya J, Yong D, Yujun B, Kai L. MiR-199a-3p affects the multi-chemoresistance of osteosarcoma through targeting AK4. BMC Cancer. 2018;18(1):631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141(4):1202–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]