Fig. 6.
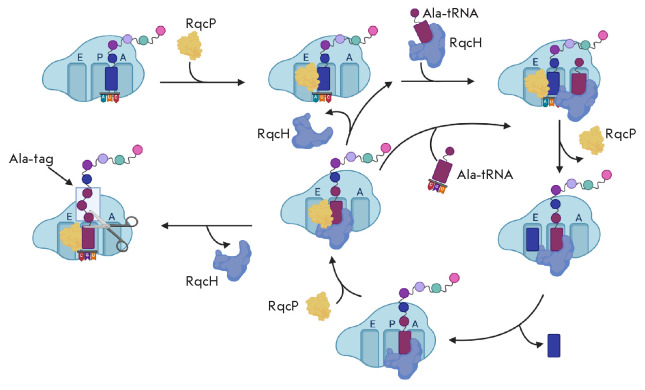
Mechanism of action of the RqcP and RqcH (YabO) proteins. RqcP binds to the 50S subunit and stabilizes tRNA at the P site [44, 45]. RqcH delivers the charged alanine tRNA to the 50S, which occupies a free A site. Further, a polypeptide chain is transferred. Then, RqcP loses its affinity to the ribosome and undergoes a translocation-like movement: in this case, the deacylated tRNA moves to the E site and the peptidyl-tRNA moves to the P site. Later, RqcP rebinds to stabilize the peptidyl-tRNA at the P site. The ribosome-bound RqcH recruits Ala-tRNA. Further, the cycle of this “elongation” can be repeated until the RqcH factor dissociates, and the polypeptide is released. The factor hydrolyzing the peptidyl-tRNA is not exactly known. Probably, it is ArfB