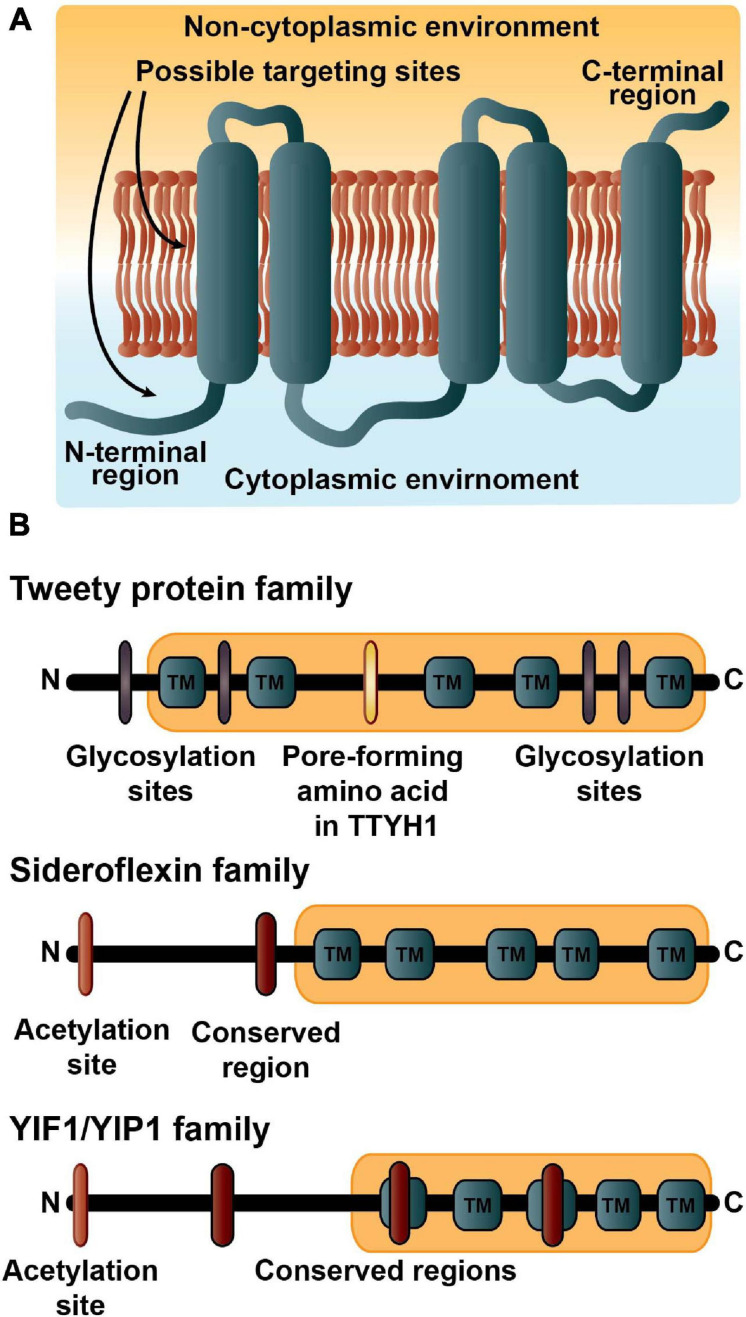
FIGURE 1.
The five transmembrane architecture. (A) The basic topology of the 5TM dataset. More than half the proteins in the dataset have the amino (N)-terminal region in the cytoplasmic environment and the carboxyl (C)-terminal in the luminal region. Many of the proteins are expected to contain targeting signals embedded in the first transmembrane region along with possibly amino acid residues in the N-terminus. (B) The domain structures and important residue modifications affecting localizations of the three major 5TM families. The description of the tweety family includes estimates of four possible glycosylation sites in purple; the important pore-forming amino acid (R165) in TTYH1 indicated in yellow (Han et al., 2019); and the Pfam tweety domain (PF04906) in light orange. The Sideroflexin family is annotated with a possible acetylation site at residue one or two and colored orange; the conserved HPDT residues are the red symbol; and the sideroflexin Pfam domain (PF03820) is in light orange. Many of the YIPF proteins have an acetylation site at residue one or two that is colored orange; three conserved motifs are indicated in red; and the YIPF Pfam domains (PF03878 and PF04893) are shown in light orange.