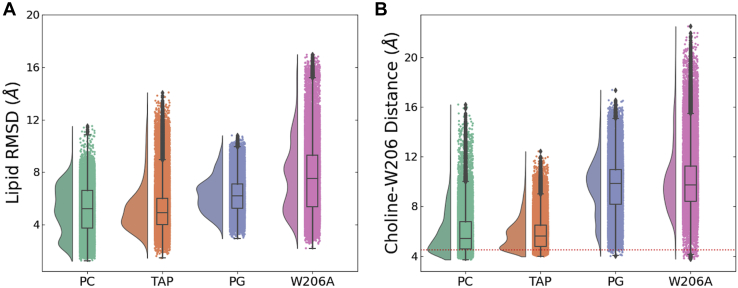
Figure 4.
Stability of different lipids at the W206-binding site.A, stability of various lipid types at the intersubunit binding site illustrated by the RMSD probability distributions and box plots. For this calculation, the Cα atoms of the M2 helix were first aligned and the RMSD was calculated for the nonhydrogen lipid headgroup atoms. The RMSD values were then averaged across the five subunit interfaces over the final 150 ns of three independent 300 ns MD trajectories. B, the role of cation-π interactions in stabilizing the lipid at the subunit interface illustrated by the distribution of distance between the choline lipid headgroup and the center of the W206 aromatic side chain. For each lipid type, the distances were averaged across the five subunit interfaces over the final 150 ns of three independent 300 ns MD trajectories. For the PG lipid without the choline group, the distances were instead calculated to the phosphorus atom and the ideal cation-π binding distance of 4.5 Å is illustrated as a dotted red line. Data are presented as a box plot