Abstract
Although cervical spinal cord injury (cSCI) disrupts bulbo-spinal serotonergic projections, partial recovery of spinal serotonergic innervation below the injury site is observed after incomplete cSCI. Since serotonin contributes to functional recovery post-injury, treatments to restore or accelerate serotonergic reinnervation are of considerable interest. Intermittent hypoxia (IH) was reported to increase serotonin innervation near respiratory motor neurons in spinal intact rats, and to improve function after cSCI. Here, we tested the hypotheses that spontaneous serotonergic reinnervation of key respiratory (phrenic and intercostal) motor nuclei: 1) is partially restored 12 weeks post C2 hemisection (C2Hx); 2) is enhanced by IH; and 3) results from sprouting of spared crossed-spinal serotonergic projections below the site of injury. Serotonin was assessed via immunofluorescence in male Sprague Dawley rats with and without C2Hx (12 wks post-injury); individual groups were exposed to 28 days of: 1) normoxia; 2) daily acute IH (dAIH28: 10, 5 minute 10.5% O2 episodes per day; 5 minute normoxic intervals); 3) mild chronic IH (IH28-5/5: 5 minute 10.5% O2 episodes; 5 minute intervals; 8 hours/day); or 4) moderate chronic IH (IH28-2/2: 2 minute 10.5% O2 episodes; 2 minute intervals; 8 hours/day), simulating IH experienced during moderate sleep apnea. After C2Hx, the number of ipsilateral serotonergic structures was decreased in both motor nuclei, regardless of IH protocol. However, serotonergic structures were larger after C2Hx in both motor nuclei, and total serotonin immunolabeling area was increased in the phrenic motor nucleus but reduced in the intercostal motor nucleus. Both chronic IH protocols increased serotonin structure size and total area in the phrenic motor nuclei of uninjured rats, but had no detectable effects after C2Hx. Although the functional implications of fewer but larger serotonergic structures are unclear, we confirm that serotonergic reinnervation is substantial following injury, but IH does not affect the extent of reinnervation.
Keywords: serotonin, raphe, spinal cord injury, cervical hemisection, intermittent hypoxia, phrenic motor neurons, intercostal motor neurons
INTRODUCTION
Over half of all spinal cord injuries (SCI) occur in the cervical region (cSCI; (NSCISC, 2020), and can cause severe respiratory impairment. Indeed, respiratory failure is the major cause of death in people with cSCI (Winslow and Rozovsky, 2003). Since most cSCI are incomplete, one promising strategy to restore breathing function is to harness intrinsic mechanisms of spinal plasticity, strengthening spared neural pathways to respiratory motor neurons (Dale-Nagle et al., 2010; Goshgarian, 2003). One key bulbo-spinal pathway necessary to enable certain forms of respiratory motor plasticity (and functional recovery) is from serotonergic raphe nuclei of the medulla (Bowker et al., 1982; Feldman et al., 2003; Jacobs and Azmitia, 1992; Mitchell et al., 2001; Skagerberg and Björklund, 1985; Steinbusch, 1981). Thus, following injury, preservation and restoration of serotonergic innervation to respiratory motor nuclei are of considerable interest.
Serotonin modulates motor neuron activity (Lindsay and Feldman, 1993; Perrier, 2016; Perrier et al., 2013) and initiates/orchestrates novel forms of spinal motor plasticity (Baker-Herman and Mitchell, 2002; MacFarlane and Mitchell, 2009; Mitchell et al., 2001). Since all CNS serotonergic neurons are located in the brainstem (Dahlström and Fuxe, 1964; Törk, 1990), cSCI disrupts descending serotonergic projections to respiratory motor nuclei (Golder and Mitchell, 2005; Perrin and Noristani, 2019; Tai et al., 1997). With time post-injury, serotonergic reinnervation partially recovers, correlating with at least some functional improvements (Camand et al., 2004; Golder and Mitchell, 2005; Hashimoto and Fukuda, 1991; Saruhashi et al., 1996; Tai et al., 1997; Zhou and Goshgarian, 2000). Despite the functional significance of this modulatory system for preservation and restoration of respiratory function, we do not yet know the full extent of spontaneous serotonergic reinnervation of phrenic or intercostal motor nuclei after chronic cSCI. Further, it is not known if spontaneous serotonergic reinnervation of ipsilateral respiratory motor nuclei after cSCI results from axon terminal sprouting in spared, crossed-spinal serotonergic projections below the site of injury, or growth of new serotonergic axons into these motor nuclei. Understanding the extent and mechanisms of serotonergic reinnervation in key respiratory motor nuclei (e.g. phrenic and intercostal) after chronic cSCI may help guide development of therapeutic strategies to restore breathing function by harnessing serotonin-dependent respiratory motor plasticity, such as therapeutic acute intermittent hypoxia (AIH; (Dale et al., 2014; Gonzalez-Rothi et al., 2015a).
AIH has emerged as a potential therapeutic tool, triggering neuroplasticity in spared neural pathways, restoring respiratory and non-respiratory motor function in both rodent models and in humans with chronic, incomplete SCI (Dale et al., 2014; Gonzalez-Rothi et al., 2015a; Hayes et al., 2014; Lovett-Barr et al., 2012). One mechanism whereby AIH enhances breathing function after SCI requires spinal serotonin receptor activation (Baker-Herman and Mitchell, 2002; Dougherty et al., 2018). Since repetitive AIH (3x per week for 10 weeks) increases serotonergic innervation of the phrenic motor nucleus of uninjured rats (Satriotomo et al., 2012), AIH may accelerate/enhance serotonergic reinnervation of phrenic and intercostal motor nuclei after chronic cSCI, enabling greater functional recovery. Although “low-dose” AIH elicits functional benefits, more intense protocols of intermittent hypoxia simulating episodes experienced during sleep apnea (chronic intermittent hypoxia; CIH) elicit dose-dependent pathology (Dale et al., 2014; Navarrete-Opazo and Mitchell, 2014b) and can undermine (Huxtable et al., 2015) or facilitate (Ling et al., 2001) the potential for AIH to elicit serotonin dependent plasticity. “High-dose” CIH is prominent in people with cSCI since nearly 80% of those with cSCI exhibit sleep apnea (Berlowitz et al., 2005; Sankari et al., 2014; Sankari et al., 2019). To optimize therapeutic AIH protocols to treat cSCI, it is essential to know the extent of spontaneous serotonergic reinnervation, and the impact of intermittent hypoxia (IH) on this process.
Here we investigate the impact of three, commonly studied IH protocols that range from low-dose, therapeutic daily AIH to more severe, pathogenic protocols simulating mild or moderate sleep apnea (Navarrete-Opazo and Mitchell, 2014b). We tested the hypotheses that: 1) spontaneous serotonergic reinnervation of phrenic and intercostal motor nuclei is nearly complete 12 weeks post C2 hemisection (C2Hx); 2) IH further enhances serotonergic reinnervation of phrenic and intercostal motor nuclei; and 3) serotonergic reinnervation is due to sprouting of existing/spared crossed-spinal serotonergic projections. Twelve weeks post-C2Hx, we report reduced numbers, but increased size of serotonergic structures innervating phrenic and intercostal motor neurons; the result is increased (phrenic) and reduced (intercostal) total serotonergic immunolabeling area within the respective motor nuclei. Reinnervation appears due to sprouting of spared crossed-spinal serotonergic projections since the number of serotonergic fibers/structures near the spinal midline below the site of injury was unchanged. Although no IH protocol affected serotonergic innervation after C2Hx, CIH increased serotonergic innervation of the phrenic motor nucleus in uninjured rats. Collectively, we demonstrate substantial serotonergic reinnervation of phrenic and intercostal motor nuclei after C2Hx, suggesting the potential to elicit serotonin-dependent respiratory motor plasticity with chronic cSCI (Dougherty et al., 2018).
METHODS
Animals
All experiments were conducted with adult male (initially ~350 grams) Sprague-Dawley rats (Envigo, Indianapolis IN, Colony 208A). Rats were housed in pairs in a controlled environment (12 hour light/dark cycles) with food and water ad libitum. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Florida Health Sciences Center. A total of 79 rats were studied, beginning at 11-12 weeks of age. Spinal intact and C2 hemisection (C2Hx) rats were randomly assigned to intermittent hypoxia exposure groups at 8 weeks post-injury (or equivalent time for uninjured rats), and exposed for 28 consecutive days to either: 1) normoxia (Nx28; n=8 intact, n=9 with C2Hx); 2) daily acute intermittent hypoxia (dAIH28, n=10 intact, n=8 with C2Hx), 3) mild chronic intermittent hypoxia (IH28-5/5, 8 hours per day; n=10 intact, n=12 with C2Hx) equivalent to an apnea/hypopnea index equivalent of ~6; and 3) moderate chronic intermittent hypoxia (IH28-2/2; n=10 intact, n=12 with C2Hx) equivalent to an apnea/hypopnea index equivalent of 15. The experimental timeline and details of the IH protocols are illustrated in Figure 1. All IH protocols are defined in detail in the section below.
Figure 1: Experimental time-line and intermittent hypoxia protocols.
A. Rats were injected with Cholera toxin subunit B (CtB) 2 weeks prior to C2 spinal hemisection (C2Hx). 8 weeks post C2Hx rats began intermittent hypoxia exposures for 28 days. B. Normoxia protocol (Nx28) consisted of 28 days of 21% O2 for 8 hours. C. Daily acute intermittent hypoxia (dAIH28) consisted of 10, 5 minute episodes of 10.5% O2 alternating with 5 minute normoxic intervals (~1.5 hours/day) for 28 days. D. Mild chronic intermittent hypoxia (IH28-5/5) consisted of 8 hours per day of 5 minute episodes of 10.5% O2 alternating with 5 minute normoxic intervals. E. Moderate chronic intermittent hypoxia (IH28-2/2) consisted of 2 minute episodes of 10.5% O2 alternating with 2 minute normoxic intervals for a total duration of 8 hours/day. Rats were randomly assigned to groups: 1) spinal intact+Nx28 (n=8); 2) spinal intact+dAIH28 (n=10); 3) spinal intact+IH28-5/5 (n=10); 4) spinal intact+IH28-2/2 (n=10); 5) C2Hx+Nx28 (n=9); 6) C2Hx+dAIH28 (n=8); 7) C2Hx+IH28-5/5 (n=12); and 8) C2Hx+IH28-2/2 (n=12).
Phrenic Motor Neuron Labeling
Anesthesia was induced with 3.5% isoflurane (in 100% O2) in a plexiglass chamber, and then maintained at 2-2.5% isoflurane via nose cone. Adequate anesthetic depth was confirmed by the absence of toe pinch and palpebral responses. To retrogradely label phrenic motor neurons, rats received bilateral intrapleural injections of Cholera toxin B fragment (CtB; 0.2% w/v CtB; dissolved in sterile H2O; Calbiochem, Billerica, MA) at least 14 days prior to C2Hx (Dale-Nagle et al., 2011; Guenther et al., 2010; Mantilla et al., 2009). 25 μL of CtB was loaded into a 25 μL Hamilton syringe attached to a 9.5 mm sterile needle for bilateral injections (2 × 12.5 μL = 25μL total per animal) at the 5th intercostal space ~6 mm deep.
C2Hx Injury
Anesthesia, C2Hx, and animal care were performed as described previously (Fuller et al., 2008; Gonzalez-Rothi et al., 2015b). Rats were induced with 3.5% isoflurane (in 100% O2) in a chamber and then maintained (2-2.5% in O2) via nose cone throughout the surgery; adequate anesthetic depth was confirmed by the absence of toe pinch and palpebral responses. Body temperature was maintained at 36.5–37.5°C with a heating pad. Artificial tears were applied to prevent eye damage (Rugby, NDC 0536-1086-91). Toenails were clipped on all limbs. The surgical site was shaved and cleaned (chlorhexidine scrub, 58829-140-01, First Priority, Inc., Elgin, IL). C2Hx was performed by making a dorsal incision on the neck and dissecting muscle to expose the C2 lamina. Following C2 laminectomy and durotomy, the left spinal cord was hemisected with a micro-knife caudal to the C2 dorsal roots. A gap (~1 mm) at the injury site was created by aspiration. At this time, visualization of C2Hx showed anatomically complete lesions with the absence of white and gray matter from the left lateral edge to the spinal cord midline. The dura was closed with 9-0 ethilon nylon suture, the overlaying muscles were closed with 3-0 Polysorb absorbable suture and the skin was closed with 9 mm stainless steel wound clips. Post-operative care included pain management with an analgesic (buprenorphine, 0.03 mg/kg, s.q., Hospira, IL) and an anti-inflammatory drug (meloxicam, 2 mg/kg., s.q. Portland, ME) given at 12 and 24-hour intervals for 2 days post-surgery. Rats received lactated Ringer’s solution (5 ml 2x/day, s.q.) and were manually fed a nutritional supplement (Diet Gel Boost; Clear H2O; Westbrook, ME) until adequate volitional drinking and eating were observed.
Intermittent Hypoxia Protocols
Eight weeks post-C2Hx (or equivalent time point in uninjured rats), rats were housed in PlexiGlass exposure cages with free access to food and water (Therapeutiq, Kansas City, MO). IH protocols were administered to the cages daily for 28 days. Normoxia control rats received continuous air flow (21% O2, 8 hours per day; Figure 1B). Rats assigned to the dAIH28 groups received 10, 5 minute episodes of 10.5% O2, alternating with 5 minute normoxic intervals for ten episodes per day (~1.5 hours/day; Figure 1C). Rats assigned to IH28-5/5 groups received 5 minute episodes of 10.5% O2, alternating with 5 minute normoxic intervals for 8 hours/day (48 episodes per day; Figure 1D). Five minute hypoxic/normoxic intervals yield 6 hypoxic episodes per hour, somewhat equivalent to mild sleep apnea (5 to 10 events per hour) (American Academy of Sleep MedicineAmerican and medicine, 1999; Young et al., 2008). Rats assigned to IH28-2/2 received 2 minute episodes of 10.5% O2, with 2 minute normoxic intervals for 8 hours/day (120 hypoxic episodes per day; Figure 1E). This protocol consisting of 15 hypoxic episodes per hour is equivalent to clinical criteria for the transition to moderate sleep apnea (American Academy of Sleep MedicineAmerican and medicine, 1999; Young et al., 2008).
Tissue Harvesting & Histology
One day after their final exposure, rats were perfused intracardially with cold 0.1M phosphate buffer saline (PBS), followed by paraformaldehyde (4% paraformaldehyde w/v in 0.1M PBS, pH 7.4). The spinal cord was harvested and then: 1) post-fixed overnight in paraformaldehyde (4% in 0.1M PBS, pH 7.4); and 2) cryoprotected at 4°C in 20% sucrose solution in 0.1M PBS for 3 days followed by 30% sucrose solution in 0.1M PBS for 3 days.
Although the C2Hx was visualized for completeness at the time of injury, we confirmed complete injuries via immunohistochemistry. Cervical spinal segments C1-C3 were embedded in paraffin, cut at 7 μm, mounted on positively charged slides (Fisherbrand, Fisher Scientific, Pittsburgh, PA), and allowed to dry overnight. Four sections per rat ranging from dorsal to ventral were stained with Luxol Fast Blue and cresyl violet. Sections were deparaffinized by heating at 60°C for 20 minutes and submerged 3 times in xylene for 5 minutes each. Sections were rehydrated by graded alcohol (100%-70%) for 2 minutes each and then stained overnight using Luxol Fast Blue (0.1 g Luxol Fast Blue in 100 mL of 95% ethanol and 0.5 mL 10% acetic acid). The following day, tissues were rinsed with 95% ethanol for 2 minutes and distilled water for 5 minutes. Differentiation was achieved by dipping the tissue in 0.5% lithium carbonate and 70% ethanol, then rehydrated by distilled water for 5 minutes. Tissues were placed in 0.1% cresyl violet for 15 minutes. Cresyl violet was rinsed by dipping tissue into distilled water twice. Tissue was then dehydrated through graded alcohol (70–100%) for 1 minute each, cleared in Histoclear twice for 2 minutes each (National Diagnostics, Atlanta, GA) and cover-slipped (Eukitt, Electron Microscope Science, PA). Injury sites were imaged using bright field at 10x zoom level (BZ-×710, Keyence Co., Osaka, Japan). Complete lateral C2Hx was verified by absence of tissue in all representative sections from midline to the lateral border (data not shown).
Cervical (C3-C5) and thoracic (T4-T6) spinal segments were transversely sectioned (40 μm thickness) using a freezing microtome (Leica SM 200R, Buffalo Grove, IL). Tissues were stored in antifreeze solution at −20°C until processed (30% glycerol, 30% ethylene glycol, 40% 0.1 M PBS, pH 7.4). A total of 24 representative tissue sections from each rat were selected and stained for serotonin and CtB-labeled phrenic and intercostal motor neurons. Starting at C3 and ending at C5, 4 tissue sections from each cervical level were selected 12 sections apart (480 μm apart) for a total of 12 cervical sections per rat. Similarly, 4 tissue sections, 12 sections apart, were selected from each T4, T5, and T6, resulting in 12 representative thoracic sections per rats. These free-floating sections were washed with 0.1M PBS (pH 7.4), then incubated in heat induced epitope retrieval (TissuePro, cat#: HIER01-32R) for 30 minutes at 85°C. Tissues were washed again with 0.1M PBS-Triton (0.1%, pH 7.4) and then incubated in a blocking solution (5% normal donkey serum (NDS, GeneTex) in 0.1M PBS-Triton (0.1%, pH 7.4) at room temperature for 60 minutes. Primary antibody staining was performed by incubating tissue sections in 5% NDS in 0.1M PBS-Triton (0.1%, pH 7.4) with anti-5-HT (1/2000, rabbit serum, Immunostar #20080) and anti-CTB (1/2500, goat serum, Millipore #227040) in 4°C overnight. The following day, tissues were washed with 0.1M PBS-Triton (0.1%, pH 7.4). Secondary antibody staining was performed by incubating tissues in 5% NDS in 0.1M PBS-Triton (0.1%, pH 7.4) with secondary antibodies conjugated to Alexa Fluor® 594 (donkey anti-rabbit 594; 1:500, Invitrogen, Ref# A11055) and Alexa Fluor® 488 (donkey anti-goat 488; 1;1000, Invitrogen, Ref# A21207) in a dark box at room temperature for 2 hours. Sections were washed with 0.1M PBS-Triton (0.1%, pH 7.4) and mounted on positively charged slides (Fisherbrand, Fisher Scientific, Pittsburgh, PA) and allowed to dry overnight. Slides were cover slipped with VectaShield Antifade Hard Set Mounting Medium (Cat# H-1400). Fluorescently labeled sections were captured using an epifluorescent microscope with 20x magnification (Keyence BZ-X700, Keyence Corporation of America, Itasca, IL). Spinal cord sides were marked prior to tissue sectioning to ensure accurate side determination during imaging. Phrenic and intercostal motor neurons were determined by CtB-positive cell labeling within the ventral horn.
Serotonin Image Acquisition
All immunofluorescently labeled tissue sections (transverse, 40 μm thickness) were imaged using an epifluorescent microscope with 20x magnification (Keyence BZ-X700, Keyence Corporation of America, Itasca, IL). At 20x magnification the aspect ratio of pixel per micron is 1:0.377. We optimized our acquisition parameters such that the background intensities are ~5% of the dynamic range with minimal (<0.1%) saturation for the positively labeled areas. All images were collected using the sectioning module with a 2D pinhole setting of 6, resulting in clear, high-resolution confocal-like images. No black balance or binning was performed. A Texas Red filter was used for 5-HT (OP-87765) and a GFP filter was used for CtB (OP-87763). Exposure times of 1/15 seconds for 5-HT and 1/5 seconds for CtB were used for imaging serotonin immunofluorescence around CtB labeled phrenic motor neurons in the cervical cord. Exposure times of 1/10 seconds for 5-HT and 1/3 seconds for CtB were used for imaging serotonin immunofluorescence around CtB labeled intercostal motor neurons in the thoracic cord. Regions for imaging were determined by CtB-positive cell labeling within the ventral horn, signifying phrenic/intercostal motor neurons. One image was taken on the left side (ipsilateral) and one on the right side (contralateral) resulting in 2 images per tissue section and 24 total images per animal (12 left, 12 right). Spinal cord side was marked prior to tissue sectioning to ensure accurate side determination during imaging. The exposure time for serotonin immunofluorescence near the cervical central commissure was 1/15 seconds. Imaging regions were determined by centering the central canal in the middle of the imaging field, resulting in 12 total images per animal.
Data Analysis
Serotonin immunofluorescence:
Serotonin immunofluorescence was quantified in each image using a custom MATLAB (MathWorks, Natick, MA, USA) code. Local background intensities within each image were calculated using a running-window median filter and were subtracted from the raw pixel intensities to obtain the background-subtracted intensities. We performed our analyses on the background-subtracted intensities. Images were binarized using a custom adaptive threshold algorithm in MATLAB as previously described (Allen et al., 2019). The adaptive threshold was calculated by constructing a pixel intensity histogram from the image. The pixel intensity corresponding to the 99th percentile was selected as the threshold to account for animal-to-animal and image-to-image differences in signal and background intensities. The images were binarized using the automated algorithm to identify the serotonin-positive areas. Two investigators compared the identified serotonin structures with the original image to ensure the accuracy of identification. Automatically identified structures significantly matched investigator analysis. Injury (or intact) and intermittent hypoxia exposure protocol remained blinded throughout analyses. We observed serotonin-positive boutons and fibers; however, we cannot differentiate between serotonin boutons and fibers using our analysis. Thus, we call these “serotonin structures”. Independent serotonin structures were determined by being larger than 4 pixels and being at least 1 pixel apart. Using these parameters, we quantified the number of structures, area per structure, total area, and intensity per structure in 24 representative images. The results in the 12 cervical and 12 thoracic sections were then averaged for each rat. Quantified parameters are described below:
Number of structures:
The number of structures was determined using in the representative images from each tissue section. The total number of structures in each section was averaged to obtain the average number of immunolabeled structures per rat.
Area per structure:
The area per structure was determined by the pixel size of each structure. The pixel numbers were multiplied by the aspect ratio of pixel per micron (pixel dimensions: 0.377 μm x 0.377 μm) to determine the area per structure using a representative image from each tissue section, and then averaged to obtain the average area of each immunolabeled structure per rat.
Total area:
The total area of serotonin-positive immunolabeling was calculated by multiplying the number of structures by the area per structure within a representative image from each tissue section and then averaged to obtain the average total area of serotonin immunolabeling per rat.
Intensity per structure:
The fluorescent intensity in each immunolabeled structure was determined within a representative image from each tissue section and then averaged per rat to obtain the intensity of each structure. All intensity measurements were normalized to the maximum possible intensity (i.e. 65536).
Identification of phrenic motor neurons:
Within the cervical C3-C5 spinal sections, the custom MATLAB code first identified CtB-labelled phrenic motor neurons versus non-positive areas and assigned them binary values (CtB-positive=1, CtB-negative=0) to determine phrenic vs. non-phrenic areas. Then, the center of gravity of the CtB-labelled phrenic motor neurons was calculated. The final region of interest was defined by a circular area with a 50 μm radius centered at the center of gravity of CtB-positive phrenic motor neurons (Seven et al., 2018). The center of gravity equation was defined as:
G: the center of gravity, pi: binary value of each pixel after thresholding (i.e. 0 for serotonin-negative or 1 for serotonin-positive), and ri (x, y): the coordinate of each pixel in the image. Center of gravity was calculated based on the distance from a standardized reference point (image origin) and binary values. One rat in the intact+Nx28 groups was excluded in the phrenic motor neuron analysis because there were no sections with CtB-labeled phrenic motor neurons.
Identification of serotonin immunolabeling in the central commissure:
The same cervical spinal tissue used to identify serotonin innervation around phrenic motor neurons was used to quantify the total area of serotonin positive pixels in the central commissures, including the dorsal and ventral commissures (lamina X). A point at the center of the central canal was selected and a circular region of interest was created with a radius of 100 μm.
Identification of intercostal motor neurons:
Within thoracic T4-T6 spinal segments, the MATLAB code first identified CtB-labelled intercostal motor neurons. Then, regions of interest were defined by a circular area with a 35 μm radius centered at the center of gravity of each CtB-positive intercostal motor neuron. If there were overlapping areas of interest within in a tissue section, serotonin positive pixels in the overlap were not counted twice. We report the raw values for serotonergic innervation of intercostal motor neurons, but we also normalized the number of serotonin structures and total area to the number of labeled intercostal motor neurons per section. Results of normalized serotonin immunolabeling around intercostal motor nuclei were consistent to the raw data, so only the raw data is presented (normalized data not shown).
Statistics:
All computed values were first averaged within the image to obtain a value for the image. Then, image values were averaged for each animal, which was used for statistical calculations. Statistical calculations were performed using JMP 11.0 (SAS Institute, Cary, NC). Serotonin immunofluorescence data were first analyzed using a mixed model with repeated measures design. The independent variables were injury (intact vs. C2Hx), IH protocol (Nx28, dAIH28, IH28-5/5, and IH28-2/2), and spinal cord side relative to injury (ipsilateral vs. contralateral as the repeated measure).
Due to the large number of comparisons within the mixed model, we were concerned that significance would be hidden within intact and C2Hx groups. To avoid type 2 statistical errors, the intact and C2Hx groups were analyzed separately. In the intact groups, we averaged the left and right sides of spinal cord and ran a one-way ANOVA with IH protocol as the independent variable (Nx28, dAIH28, IH28-5/5, and IH28-2/2). In C2Hx groups, we ran a two-way repeated measures ANOVA with IH protocol (Nx28, dAIH28, IH28-5/5, and IH28-2/2) and spinal cord side relative to injury (ipsilateral vs. contralateral as the repeated measure) as the independent variables.
Thus, we report significant interactions in all 3 analyses for each for each outcome measure of serotonin immunofluorescence within the phrenic and intercostal motor nuclei: 1) overall Mixed Model, 2) one-way ANOVA for intact groups, and 3) two-way repeated measures ANOVA for C2Hx groups.
Serotonin immunofluorescence crossing the central commissure was analyzed using a two-way ANOVA. The independent variables were injury (intact vs. C2Hx) and protocol (Nx28 vs. dAIH28 vs. IH28-5/5 vs. IH28-2/2). A significance level of 0.05 was set for all statistical comparisons. When significant differences were observed, individual comparisons were made based on the Fisher Least Significance Difference post hoc test. All data are displayed as mean ± standard error of the mean.
RESULTS
Serotonergic Innervation of Phrenic Motor Nuclei
Serotonin innervation was quantified within a 50 μm radius around CtB-labeled phrenic motor neurons. We observed prominent differences in the size of serotonergic structures after C2Hx, although there were no obvious differences in the total area of immunoreactive pixels in the phrenic motor nucleus. Serotonin projections in intact rats and contralateral to C2Hx had a thin and continuous appearance, somewhat like “beads on a string.” Ipsilateral to C2Hx, serotonin structures were less abundant but larger, giving an overall appearance of greater serotonin immunolabeling, regardless of IH protocol (Figure 2; refer to Figure 1 for description of IH protocols). No obvious differences in serotonin immunofluorescence were observed between different cervical spinal segments.
Figure 2: Representative images of serotonergic innervation in the phrenic motor nucleus.
Representative images from uninjured rats, and rats 12 weeks post-C2Hx exposed to 28 days of normoxia. Images illustrate serotonin (red), often described as looking like “beads on a string”, and Cholera toxin subunit B-labeled phrenic motor neurons (green). Images from left to right are representative samples from the left and right side of the cervical spinal cord of intact rats, and the ipsilateral and contralateral sides of the cervical spinal cord from rats 12 weeks post-C2Hx. Images were taken at 20x magnification with the phrenic motor nucleus at the center. The white scale bars represent a distance of 100 μm.
Regardless of IH protocol, there was a significant reduction in serotonergic structure number in the phrenic motor nucleus after injury vs. intact rats regardless of intermittent hypoxia protocol (Mixed Model; interactions injury and injury vs. side p<0.0001; side p=0.0008; all other interactions p>0.05; Figure 3A). The number of structures ipsilateral to injury was reduced by 30% in the (Fisher LSD post hoc analysis; C2Hx ipsilateral vs. all p<0.0001) and there was a 12% reduction in the contralateral phrenic nucleus versus intact rats (Fisher LSD post hoc analysis; C2Hx contralateral vs. intact p=0.048). There was no difference between the left and right sides of intact rats (Fisher LSD post hoc analysis; p=0.292). Within C2Hx rats, the number of serotonergic structures ipsilateral to injury was 20% less than the contralateral side (two-way repeated measures ANOVA; p<0.0001). IH protocol had no effect on the number of serotonin structures near CtB-labeled phrenic motor neurons in injured or uninjured rats (Mixed Model; p=0.1377; one-way ANOVA for intact; p=0.3919; two-way repeated measures ANOVA for C2HX; p= 0.1972).
Figure 3: Serotonergic innervation of the phrenic motor nucleus: effects of injury and IH protocol.

Serotonin innervation in the phrenic motor nucleus was quantified in uninjured rats (left) and rats 12 weeks post-C2 hemisection (C2Hx; right) exposed to 28 days of intermittent hypoxia (IH) protocols (Nx28, white; dAIH28, light gray; IH28-5/5, dark gray; IH28-2/2, black). A. Number of serotonin structures. C2Hx significantly reduced the number of serotonin structures ipsi- and contralateral to C2Hx versus uninjured rats (p<0.0001 and p<0.05, respectively). Post-C2Hx, ipsilateral serotonin structure number was also significantly lower vs. contralateral side (p<0.0001). B. Size of serotonin structures. C2Hx significantly increased area of serotonin structures ipsilateral to injury versus uninjured or contralateral side (p<0.0001). There were no IH effects after C2Hx. We averaged the left and right sides of uninjured rats and ran a one-way ANOVA by IH protocol. IH28-5/5 and IH28-2/2 rats had significantly greater areas per structure vs. dAIH28 or Nx28 (IH28-2/2 vs. Nx28 p=0.0024, IH28-2/2 vs. dAIH28 p=0.0034, IH28-5/5 vs. Nx28 p=0.0087, IH28-5/5 vs. dAIH28 p=0.0134). C. Total area of serotonin immunolabeling. C2Hx significantly increased the total area of serotonin immunolabeling ipsilateral to injury versus uninjured rats or contralateral side (p<0.0001). There were no IH effects after C2Hx. We averaged the left and right sides of uninjured rats and ran a one-way ANOVA by IH protocol. IH28-5/5 and IH28-2/2 protocols had significantly greater areas per structure than Nx28 (IH28-2/2 vs. Nx28 p=0.0179, IH28-5/5 vs. Nx28 p=0.0262). All other data were analyzed via repeated measures Mixed Model ANOVA; injury (intact vs. C2Hx) and IH protocol (Nx28, dAIH28, IH28-5/5 and IH28-2/2) were between subject variables; side (left/ipsilateral vs. right/contralateral) was the within subject repeated measure. Differences were considered significant if p<0.05. Bars denote means ± 1 SEM with the individual rats denoted as data points.
Next, we quantified the average area of each serotonin labeled structure near CtB-labeled phrenic motor neurons (Figure 3B). Ipsilateral to C2Hx, serotonin structures were significantly enlarged (Mixed model; interaction of injury, side, and injury vs. side p<0.0001); the average size of each structure was nearly double (90% increase) that in uninjured rats, regardless of IH protocol (Fisher LSD post hoc analysis; C2Hx ipsilateral vs. intact ipsilateral and contralateral p<0.0001; all other interactions p>0.05; Two-way repeated measures ANOVA of C2Hx side; p<0.0001). Within the intact groups, we averaged the left and right spinal cord sides and found that IH28-5/5 and IH28-2/2 had significantly greater areas per structure (~13%) versus dAIH28 and Nx28 (one-way ANOVA; p=0.0023; Fisher LSD post hoc analysis; IH protocol; IH28-2/2 vs. Nx28 p=0.0024, IH28-2/2 vs. dAIH28 p=0.0034, IH28-5/5 vs. Nx28 p=0.0087, IH28-5/5 vs. dAIH28 p=0.0134; IH28-2/2 vs. IH28-5/5 p=0.5904; dAIH vs. Nx p=0.6791). In injured rats alone, there was no similar effect of IH on the size of serotonin structures (two-way repeated measures ANOVA for C2Hx on IH protocol; p=0.9623).
The total area of serotonin immunolabeling actually increased post-C2Hx compared to uninjured rats (Mixed Model; interaction injury p=0.0249; side and injury vs. side p<0.0001; all other interactions p>0.05; Figure 3C). Ipsilateral to C2Hx, the total area of serotonergic immunolabeling was 30% greater versus intact (Fisher LSD post hoc analysis; ipsilateral to injury vs. all p<0.0001; all other interactions p>0.05). Ipsilateral structures were also significantly larger versus contralateral side (~41%; two-way repeated measures ANOVA of C2Hx side; p<0.0001). However, IH had no effect on the total area of serotonin immunolabeling after C2Hx (two-way repeated measures ANOVA; p=0.4104). In the uninjured rats, when the left and right sides were averaged and analyzed via one-way ANOVA (by IH protocol), IH28-5/5 and IH28-2/2 had significantly greater total areas versus Nx28, about a 25% increase (Fisher LSD post hoc analysis; IH28-2/2 vs. Nx28 p=0.0179, IH28-5/5 vs. Nx28 p=0.0262).
Serotonin Positive Immunolabeling in the Central Commissure
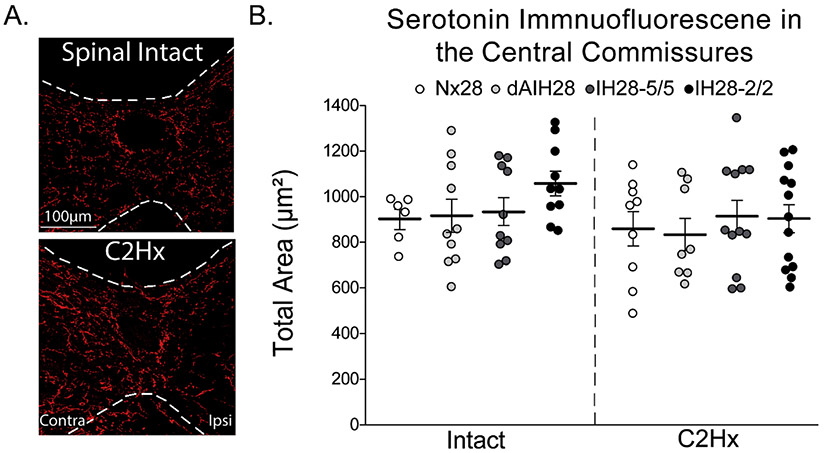
Immunofluorescent labeling was used to identify serotonergic structures at the cervical central commissure in the same sections used to determined serotonergic reinnervation in the phrenic motor nucleus. This analysis was used as a proxy to determine if ipsilateral serotonin structures observed after C2Hx were due to sprouting of existing (spared) crossed spinal pathways versus possibly new contralateral projections crossing the midline. All spinal sections studied had positive serotonin immunolabeling in this midline region. On visual inspection, there were no obvious differences in serotonin labeling of the central commissure between groups, or between different cervical spinal segments (Figure 4A). Within all C2Hx groups, we noticed greater immunolabeling contralateral versus ipsilateral to injury. We did not quantify side differences in serotonin immunofluorescence around the central canal since we were interested in the central commissure as a whole. Thus, we quantified the total area of serotonin-positive immunolabeling in a region of interest around the central canal (Figure 4B). There was no statistically significant difference in the total area of serotonin immunopositive labeling surrounding the central commissures of uninjured versus injured rats, regardless of IH protocol (two-way ANOVA; p=0.4648; refer to Figure 1 for description of IH protocols). Although there were no statistically significant effects of IH protocol, there was slightly more serotonin immunolabeling in the central commissures of uninjured rats treated with IH28-2/2.
Figure 4: Total area of serotonin immunopositive labeling at cervical central commissures.
A. Representative images of serotonergic fibers near central commissure of cervical spinal sections (left). Spinal intact (top) and C2 hemisection (C2Hx; bottom) rats were exposed to 28 consecutive days of normoxia. Dotted white lines represent gray and white matter border. Serotonin (red) mostly occurs in gray matter and exhibits the typical “beads on a string” morphology. Images were taken at 20x magnification with the central canal at the center. The white scale bars represent a distance of 100 μm. B. Quantitative analysis of serotonergic fibers at central commissures (right). Neither C2Hx nor any IH protocol (Nx28, white; dAIH28, light gray; IH28-5/5, dark gray; IH28-2/2, black) had an effect on total area of serotonergic innervation in cervical central commissures. Data were analyzed via two-way ANOVA (p=0.4648) with injury (intact vs. C2Hx) and IH protocol (Nx28, dAIH28, IH28-5/5, and IH28-2/2) as independent variables. Bars denote means ± 1 SEM with individual rats as individual data points.
Serotonergic Innervation of Intercostal Motor Nuclei
Serotonin innervation was quantified within a 35 μm radius around CtB-labeled intercostal motor neurons. Upon first glance, we did not notice obvious differences in serotonergic innervation between IH protocols in either uninjured or injured rats. However, we did observe obvious differences in serotonergic innervation after C2Hx, similar to the phrenic motor pool. In intact rats, or contralateral to C2Hx in injured rats, serotonin projections appeared normal (i.e. thin and continuous; “beads on a string”). Ipsilateral to C2Hx, serotonin structures were less abundant and thicker/larger, regardless of IH protocol (Figure 5; refer to figure 1 for IH protocol description). No obvious differences in serotonin immunofluorescence were observed between thoracic segments.
Figure 5: Representative images of serotonergic innervation in intercostal motor nuclei.
Representative images from uninjured rats, and rats 12 weeks post-C2Hx exposed to 28 days of normoxia. Images illustrate serotonin (red), often described as looking like “beads on a string”, and cholera toxin subunit B-labeled phrenic motor neurons (green). Images from left to right are representative samples from the left and right side of the thoracic spinal cord of intact rats, and the ipsilateral and contralateral sides of the thoracic spinal cord from rats 12 weeks post-C2Hx. Images were taken at 20x magnification with the phrenic motor nucleus at the center. The white scale bars represent a distance of 100 μm.
The number of serotonin structures around intercostal motor neurons was reduced 12 weeks post-C2Hx (Mixed Model; interactions injury, side, and injury vs. side p=<0.0001; Figure 6A). There were significantly fewer structures (~44%) ipsilateral to injury versus the contralateral side or uninjured rats (Fisher LSD post hoc analysis, p<0.0001; two-way repeated measures analysis for C2Hx p<0.0001). The number of serotonin structures was unaffected by IH in injured or uninjured rats (Mixed Model; interaction protocol p=0.2507; one-way ANOVA for intact groups by protocol; p=0.2313; two-way repeated measures ANOVA for C2Hx groups; p=0.7245). The size of serotonin structures was significantly larger on the ipsilateral side post-C2Hx versus the contralateral side or uninjured rats (~56%; Figure 6B; Mixed Model; interaction of injury, side, and injury vs. side p<0.0001; Fisher LSD post-hoc analysis; C2Hx ipsilateral vs all p<0.0001; two-way repeated measures ANOVA; C2Hx ipsilateral vs. contralateral p<0.0001). There was no effect of IH protocol on the average size of serotonergic structures in intact or C2Hx rats (Mixed Model; p=0.347; one-way ANOVA on intact; p=0.2677; two-way repeated measures ANOVA on C2HX; p=0.1093).
Figure 6: Serotonergic innervation of the intercostal motor nucleus.

Serotonin innervation in intercostal motor nuclei was quantified in uninjured rats (left) and rats 12 weeks post-C2 hemisection (C2Hx; right) exposed to 28 days of IH protocols (Nx28, white; dAIH28, light gray; IH28-5/5, dark gray; IH28-2/2, black). A. Number of serotonin structures. C2Hx significantly reduced the number of serotonin structures ipsilateral to injury versus uninjured rats and contralateral side (p<0.0001 for all). No IH protocol significantly affected the number of serotonin structures innervating intercostal motor nuclei in uninjured rats or rats 12 weeks post-C2Hx. B. Size of serotonin structures. C2Hx significantly increased the area of individual serotonergic structures ipsilateral to injury versus uninjured rats and contralateral side (p<0.0001 for all). IH had no effects on uninjured rats or rats 12 weeks post-C2Hx. C. Total area of serotonin immunolabeling. C2Hx reduced the total area of serotonin immunolabeling ipsilateral to injury versus uninjured rats or the contralateral side (p<0.0001 for all). There were no IH effects on total area of intercostal motor nuclei in uninjured rats. All data were analyzed with a repeated measures Mixed Model ANOVA; injury (intact vs. C2Hx) and IH protocol (Nx28, dAIH28, IH28-5/5 and IH28-2/2) were between subject variables; side (left/ipsilateral vs. right/contralateral) was the repeated measure. Differences were considered significant if p<0.05. Bars denote means ± 1 SEM with the individual rats as individual data points.
The total area of serotonin immunolabeling around intercostal motor neurons (Figure 6C) exhibited complex differences post-injury. There was an interaction of protocol vs. injury vs. side on total area of serotonin immunolabeling (Mixed Model; p=0.0344; interaction of injury and side alone p=0.0094 and p=0.0082 respectively; all other interactions p>0.05). The total area ipsilateral to C2Hx was 16% lower versus the contralateral side or in intact rats regardless of IH protocol (Fisher LSD post hoc analysis; C2Hx ipsilateral vs. C2Hx contralateral, intact ipsilateral, and intact contralateral all p<0.05; two-way repeated measures ANOVA on C2HX ipsilateral vs. contralateral; p=0.0116). Although not statistically significant, ipsilateral to C2Hx, exposure to dAIH28 increased (~10%) total serotonin immunolabeling area versus Nx28. There were no effects of IH protocol on total serotonin area within the contralateral side or uninjured rats (Fisher LSD post hoc analysis for Mixed Model; p>0.05 for all comparisons; one-way analysis on intact; p=0.7548; two-way repeated measures analysis for C2Hx; p=0.7617).
Intercostal motor nuclei are spaced along the thoracic spinal cord and do not cluster like phrenic motor neurons. Thus, to identify possible differences, and bias, in intercostal motor neurons, we counted the number of CtB-labeled intercostal motor neurons in each thoracic spinal section. The average number of CtB-labeled intercostal motor neurons varied from 1-5 neurons per 40μm section of thoracic spinal tissue (data not displayed). We found no differences in intercostal motor neuron numbers between injured and uninjured rats (Mixed Model; p=0.421), and there were no effects of IH protocol (Mixed Model; p=0.956). In addition to quantifying the raw serotonin structure numbers and total area, we normalized serotonin structure number and total area to the average number of CtB-labeled intercostal motor neurons per thoracic section to account for slight variations in the number of intercostal motor neurons per section. The overall results were comparable between the raw and normalized data, so we only report the raw data.
Serotonin Immunofluorescent Intensity Around Phrenic and Intercostal Motor Nuclei
We quantified staining intensity in serotonergic structures in the phrenic and intercostal motor nuclei (Figure 7) despite known limitations in using immunofluorescence as an indicator of antigen concentration (Fritschy, 2008; Matos et al., 2010). In the phrenic motor nucleus, average serotonin staining intensity within serotonin-positive structures was decreased by C2Hx (Mixed Model; interaction of injury p=0.0003, side p<0.0001, and injury vs. side p<0.0001; Figure 7A). Ipsilateral to C2Hx, serotonin structures had 36% lower fluorescent intensity compared to the contralateral side or intact rats (Fisher LSD post hoc analysis of ipsilateral; all p<0.0001; Two-way repeated measures ANOVA for C2Hx ipsilateral vs. contralateral; p<0.0001). IH had no impact on serotonin labeling intensity after C2Hx (two-way repeated measures ANOVA; p=0.443). In uninjured rats, we averaged left and right sides and ran a one-way ANOVA for IH protocol; in this analysis, dAIH28 had significantly greater structure intensity (27%) versus Nx28 (Fisher LSD post hoc analysis; p=0.044). There were no significant effects of mild (IH28-5/5) or moderate (IH28-2/2) CIH protocols in intact rats.
Figure 7: Immunoreactive serotonin staining intensity within immunopositive structures.
Fluorescent intensity in immunopositive structures of the phrenic motor nucleus was quantified in uninjured rats (left) and rats 12 weeks post-C2 hemisection (C2Hx; right) exposed to 28 days IH protocols (Nx28, white; dAIH28, light gray; IH28-5/5, dark gray; IH28-2/2, black). A. Serotonin labeling intensity in phrenic motor nucleus. C2Hx significantly reduced the intensity of serotonin positive structures ipsilateral to injury versus uninjured rats and contralateral side (p<0.0001). There was no IH effect on staining intensity after C2Hx. We averaged the left and right sides of uninjured rats and ran a one-way ANOVA by IH protocol. In uninjured rats, dAIH28 significantly increased staining intensity per structure versus Nx28 (p=0.0444). B. Serotonin labeling intensity in intercostal motor nuclei. C2Hx significantly reduced the intensity of serotonin staining within structures ipsilateral to C2Hx versus uninjured rats or contralateral side (p<0.0001). There were no IH effects on serotonin structure intensity in uninjured rats or rats post- C2Hx. All data were analyzed with a repeated measures Mixed Model ANOVA; injury (intact vs. C2Hx) and IH protocol (Nx28, dAIH28, IH28-5/5 and IH28-2/2) were between subject variables; side (left/ipsilateral vs. right/contralateral) was the within subject repeated measure. Differences were considered significant if p<0.05. Data are displayed in arbitrary units (A.U.). Bars denote means ± 1 SEM with the individual rats as individual data points.
In the intercostal region, serotonin positive structures had ~59% lower average intensities ipsilateral to C2Hx compared to the contralateral side and intact rats (Mixed Model; interactions of injury, side, and injury vs. side p<0.0001; Figure 7B). There was no effect of any IH protocol on the intensity of immunoreactivity within serotonin structures around intercostal motor nuclei of injured or uninjured rats (Mixed Model; p=0.0988). In the intact group alone, there was no effect of IH protocol (one-way ANOVA; p=0.254). After C2Hx, ipsilateral intensities were significantly less than contralateral and there were no effects of IH protocols (two-way repeated measures ANOVA; p<0.0001 and p=0.286 respectively).
DISCUSSION
We investigated spontaneous serotonergic reinnervation of the phrenic and intercostal motor nuclei following cervical spinal hemisection, a frequently studied model of cSCI. Further, we investigated the impact of multiple, frequently studied IH protocols on serotonergic innervation in both injured and uninjured rats. Twelve weeks post-C2Hx, spontaneous reinnervation of the ipsilateral phrenic and intercostal motor nuclei was substantial, characterized by reduced numbers but larger serotonergic structures. Total serotonin immunolabeling was actually increased (phrenic) or restored (intercostal) in these motor nuclei. Thus, with time post-injury, spontaneous serotonergic reinnervation of respiratory motor nuclei is robust, and likely sufficient to contribute meaningfully to serotonin-dependent respiratory motor plasticity. This knowledge is essential as we work to harness such plasticity as a therapeutic modality to restore breathing ability after cSCI (Dale et al., 2014; Gonzalez-Rothi et al., 2015a)
CIH for 28 days simulating mild (IH-5/5) or moderate (IH-2/2) sleep apnea (protocols described in Figure 1) increased the size of serotonergic structures in the phrenic motor nucleus of uninjured rats, although the IH protocol with therapeutic promise, dAIH28, had no detectable effect. In contrast, none of the IH protocols studied had any effect on serotonergic innervation of the phrenic motor nucleus in C2Hx rats. However, in the intercostal motor nuclei, dAIH28 increased serotonergic innervation ipsilateral to C2Hx, suggesting at least some potential of daily AIH to promote serotonin-dependent intercostal motor plasticity following SCI. Although the exact protocol that will elicit the greatest phrenic and intercostal plasticity after SCI remains unclear, this study is a critical step in the optimization of IH protocols to enhance respiratory plasticity.
Serotonergic Reinnervation after C2Hx
Spinal serotonin arises from the raphe nucleus in the medulla, which descends mostly to the ipsilateral spinal cord, with contralateral projections that decussate at multiple spinal segmental levels (Bowker et al., 1981; Liang et al., 2015). C2Hx severs ipsilateral descending axons from medullary serotonergic neurons innervating the phrenic and intercostal motor nuclei (Golder and Mitchell, 2005; Perrin and Noristani, 2019; Tai et al., 1997). Previous studies report only partial serotonergic reinnervation of phrenic motor nuclei by 8 weeks post-C2Hx. Thus, the nearly complete restoration of total serotonin immune-reactive area within these motor nuclei 12 weeks post-injury is notable, although the functional significance of fewer but larger serotonin immunoreactive structures remains unclear. Regardless, the extent of recovery is indicative of the potential for recovery of serotonin-dependent plasticity with time post-injury (Dougherty et al., 2018; Navarrete-Opazo et al., 2017; Navarrete-Opazo et al., 2015).
The diameter of serotonin terminals in the phrenic motor nucleus of cats ranges from 1-3μm (Pilowsky et al., 1990). In the present study, serotonin-positive areas were observed were not circular but rather exhibited other shapes such as ellipses or long fibrillary projections. Thus, we report the area of serotonin structures around phrenic motor neurons, which ranged from 1 to 2 μm2 in intact rats. If we were the calculate “equivalent diameters” from our area measurements, we obtain 1.1 to 1.6 μm which fits well within the range they reported by Pilowsky and colleagues (1990). We found that after C2Hx, ipsilateral serotonin structures nearly doubled in size to ~3 μm2. These data support literature accounts that serotonin axons enlarge and that their terminals swell during sprouting (Mamounas et al., 2000; Mamounas et al., 1995). Other accounts suggest that indirect injuries enhance serotonergic innervation of the phrenic motor nucleus; for example, 26 days after bilateral cervical dorsal rhizotomy, the number of serotonergic terminals in the phrenic motor nucleus is increased (Kinkead et al., 1998).
Thirty days following an incomplete C2 lateralized hemisection, the number of serotonergic terminals in the phrenic motor nucleus was reported to increase, as was the number of active zones between serotonin terminals and phrenic motor neurons (Tai et al., 1997). Although we found reduced numbers of serotonergic terminals 12 weeks post-injury, possibly due to more complete spinal hemisections, increased size post-C2Hx enhanced the overall area of serotonin innervation within the phrenic motor nucleus. Considerably less is known about serotonin reinnervation of the intercostal motor nuclei, but our data suggests a similar pattern to the phrenic motor nucleus with the exception that the swelling of serotonin terminals is less robust.
The source of serotonin structures reinnervating the phrenic motor nucleus is not completely clear, but suggestive evidence leads us to conclude that spared terminals ipsilateral to injury sprout and hypertrophy. After C2Hx, whereas descending ipsilateral projections are disrupted, crossed spinal projections below the site of injury remain intact. Since there was more serotonin immunofluorescence in the ipsilateral phrenic motor pool 12 weeks post-C2Hx, we determined if this was due to new serotonin projections crossing the cervical central commissure. The lack of change in total area of serotonin immunoreactive fibers crossing the spinal midline strongly suggests that reinnervation results from sprouting of existing pathways versus the growth of new serotonergic projections across the spinal midline. Our findings confirm a previous report that new serotonergic projections fail to cross the spinal midline after SCI (Saruhashi et al., 1996), and are consistent with an electron microscopy study demonstrating sprouting of serotonin terminals and new synapse formation with phrenic motor neurons after C2Hx (Tai et al., 1997).
Because this study concerned anatomical characteristics of serotonergic innervation in the phrenic and intercostal nuclei, the functional significance of abnormal serotonin immunoreactive structure number and size remains uncertain. Although these results are consistent with spontaneous return of serotonergic function post-injury, further studies are needed to verify function in these abnormally large structures.
Effects of IH on Serotonergic Innervation in Uninjured Rats
The observations that IH28-5/5 and IH28-2/2, but not dAIH28, increased size of phrenic serotonergic structures in uninjured rats has important implications concerning the ability of IH preconditioning to enhance serotonergic function. For example, one week of moderate CIH (5 minute hypoxia intervals for 12 hours/night) enhances serotonin-dependent AIH-induced phrenic long-term facilitation in normal rats (Ling et al., 2001). In that study, the mechanism of enhanced phrenic long-term facilitation was serotonin-dependent, but they did not investigate IH effects on serotonergic innervation. Our finding that similar CIH (5 minute hypoxia intervals for 8 hours/day) enlarges serotonin structures in the phrenic motor nucleus of uninjured rats suggest enhanced serotonin release, enabling greater serotonin-dependent plasticity (Ling et al., 2001; MacFarlane and Mitchell, 2009). To our knowledge, CIH-enlarged serotonergic terminals in respiratory motor nuclei have not been reported previously.
Lower doses of repetitive AIH (10, 5 minute episodes, 3x/week, 10 weeks) also enhance serotonergic innervation in the phrenic motor nucleus (Satriotomo et al., 2012) and AIH-induced phrenic long-term facilitation (MacFarlane et al., 2018) in uninjured rats. However, we did not observe similar anatomical findings following dAIH28 in the present study (i.e. there was no increase in serotonergic innervation). These prior studies differed in the total duration of repetitive AIH exposure (10 vs. 4 weeks), details of AIH exposure (daily versus 3x per week), and sub-strains of Sprague Dawley rats used (Envigo versus Taconic).
Although fluorescent intensity measurements correlate with serotonin concentration, it is not a linear relationship (Matos et al., 2010) and we must remain tentative with our conclusions. Even so, our observation that dAIH28 increased serotonin staining intensity within serotonergic structures of the phrenic motor nucleus of uninjured rats may help explain differences in our findings versus those of Satriotomo and colleagues (2012). Since Satriotomo and colleagues (2012) quantified the number of serotonin-positive pixels (i.e. total area) to represent serotonergic innervation, greater serotonin staining intensity within structures could increase the apparent number of immunopositive pixels, bringing sub-threshold but existing structures across the detection threshold, leading to increased counts. However, at this point, conclusions regarding differences in our findings must remain tentative pending direct verification.
In contrast to the phrenic motor nucleus, IH had no apparent impact on serotonergic innervation of intercostal motor nuclei in uninjured rats. Reasons for these differences between intercostal versus phrenic motor nuclei are unclear.
Lack of IH Effects on Serotonergic Reinnervation 12 Weeks Post-C2Hx
Although we originally predicted that CIH would enhance and/or accelerate serotonergic recovery after C2Hx, it had no significant impact on phrenic or intercostal serotonergic innervation in injured rats. Since serotonin reinnervation is already robust at this time, we cannot rule out the possibility that CIH accelerates, but does not otherwise enhance recovery. Further, the reasons that IH increases serotonergic innervation in intact, but not injured rats remains unclear.
Although the serotonergic structures were larger in ipsilateral intercostal motor nuclei post-C2Hx, the total area of serotonin immunolabeling was reduced, due to reductions in serotonin structure number. One significant finding was that dAIH28 restored intercostal serotonergic innervation to normal levels ipsilateral to C2Hx, suggesting dAIH preconditioning may enhance serotonergic function after SCI. These results suggest that serotonin targeted therapies may be effective post-SCI, and may be enhanced by the cumulative effects of repeated AIH. Indeed, AIH elicits profound intercostal plasticity (Navarrete-Opazo and Mitchell, 2014a) and repeated AIH improves intercostal activity post-cSCI (Navarrete-Opazo et al., 2015). Our current results verify that serotonin-dependent recovery of intercostal function is possible after cervical SCI.
CONCLUSION
We report substantial spontaneous serotonergic reinnervation of the phrenic and intercostal motor nuclei 12 weeks post-C2Hx, characterized by reduced numbers of larger serotonergic varicosities surrounding phrenic and intercostal motor neurons. Although the functional impact of increased serotonergic structure size is unclear, our results demonstrate that substantial serotonergic function may be possible after chronic cervical SCI. Thus, serotonin-targeted therapies may be possible to enhance respiratory function after SCI, such as repetitive low-dose AIH. Indeed, repetitive AIH elicits spinal respiratory motor plasticity and functional recovery in both rodent models and humans with chronic SCI (Dale et al., 2014; Gonzalez-Rothi et al., 2015a).
Highlights:
Extensive serotonergic reinnervation of phrenic and intercostal motor nuclei 12 weeks following cervical hemisection
Fewer, but larger serotonergic structures in phrenic and intercostal motor nuclei post-injury
Ipsilateral phrenic reinnervation results from sprouting in spared crossed-spinal serotonergic pathways
FUNDING:
This work was supported by the National Institutes of Health (OT2OD023854; HL69064 & HL147554) and the University of Florida McKnight Brain Institute (G.S. Mitchell & M.C. Ciesla). L.L. Allen supported by NICHD T32-HD043730; E.J. Gonzalez-Rothi supported by K12-HD055929. A.E. Holland and J.V. Santiago supported by the American Physiological Society (UGREF, STRIDE). The authors declare no competing financial interests.
Non-standard Abbreviations and Acronyms:
- cSCI
cervical spinal cord injury
- IH
intermittent hypoxia
- AIH
acute intermittent hypoxia
- CIH
chronic intermittent hypoxia
- C2Hx
C2 spinal hemisection
- CtB
cholera toxin B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES:
none
REFERENCES:
- Allen LL, Seven YB, Baker TL, Mitchell GS, 2019. Cervical spinal contusion alters Na. Respir Physiol Neurobiol 261, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American, medicine, a.o.s., 1999. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22, 667–689. [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS, 2002. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22, 6239–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlowitz DJ, Brown DJ, Campbell DA, Pierce RJ, 2005. A longitudinal evaluation of sleep and breathing in the first year after cervical spinal cord injury. Arch Phys Med Rehabil 86, 1193–1199. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Westlund KN, Coulter JD, 1981. Origins of serotonergic projections to the spinal cord in rat: an immunocytochemical-retrograde transport study. Brain Res 226, 187–199. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Westlund KN, Sullivan MC, Coulter JD, 1982. Organization of descending serotonergic projections to the spinal cord. Prog Brain Res 57, 239–265. [DOI] [PubMed] [Google Scholar]
- Camand E, Morel MP, Faissner A, Sotelo C, Dusart I, 2004. Long-term changes in the molecular composition of the glial scar and progressive increase of serotoninergic fibre sprouting after hemisection of the mouse spinal cord. Eur J Neurosci 20, 1161–1176. [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K, 1964. Localization of monoamines in the lower brain stem. Experientia 20, 398–399. [DOI] [PubMed] [Google Scholar]
- Dale EA, Ben Mabrouk F, Mitchell GS, 2014. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology (Bethesda) 29, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Satriotomo I, Lovett-Barr MR, Vinit S, Mitchell GS, 2010. Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann N Y Acad Sci 1198, 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Satriotomo I, Mitchell GS, 2011. Spinal vascular endothelial growth factor induces phrenic motor facilitation via extracellular signal-regulated kinase and Akt signaling. J Neurosci 31, 7682–7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty BJ, Terada J, Springborn SR, Vinit S, MacFarlane PM, Mitchell GS, 2018. Daily acute intermittent hypoxia improves breathing function with acute and chronic spinal injury via distinct mechanisms. Respir Physiol Neurobiol 256, 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE, 2003. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26, 239–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, 2008. Is my antibody-staining specific? How to deal with pitfalls of immunohistochemistry. Eur J Neurosci 28, 2365–2370. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ, 2008. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol 211, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS, 2005. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci 25, 2925–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Lee KZ, Dale EA, Reier PJ, Mitchell GS, Fuller DD, 2015a. Intermittent hypoxia and neurorehabilitation. J Appl Physiol (1985) 119, 1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Rombola AM, Rousseau CA, Mercier LM, Fitzpatrick GM, Reier PJ, Fuller DD, Lane MA, 2015b. Spinal interneurons and forelimb plasticity after incomplete cervical spinal cord injury in adult rats. J Neurotrauma 32, 893–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG, 2003. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol (1985) 94, 795–810. [DOI] [PubMed] [Google Scholar]
- Guenther CH, Vinit S, Windelborn JA, Behan M, Mitchell GS, 2010. Atypical protein kinase C expression in phrenic motor neurons of the rat. Neuroscience 169, 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Fukuda N, 1991. Contribution of serotonin neurons to the functional recovery after spinal cord injury in rats. Brain Res 539, 263–270. [DOI] [PubMed] [Google Scholar]
- Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD, 2014. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology 82, 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Smith SM, Peterson TJ, Watters JJ, Mitchell GS, 2015. Intermittent Hypoxia-Induced Spinal Inflammation Impairs Respiratory Motor Plasticity by a Spinal p38 MAP Kinase-Dependent Mechanism. J Neurosci 35, 6871–6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC, 1992. Structure and function of the brain serotonin system. Physiol Rev 72, 165–229. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS, 1998. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci 18, 8436–8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Wang S, Francis R, Whan R, Watson C, Paxinos G, 2015. Distribution of raphespinal fibers in the mouse spinal cord. Mol Pain 11, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay AD, Feldman JL, 1993. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol 461,213–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Mitchell GS, 2001. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci 21, 5381–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS, 2012. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci 32, 3591–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS, 2009. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol 587, 5469–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Vinit S, Mitchell GS, 2018. Enhancement of phrenic long-term facilitation following repetitive acute intermittent hypoxia is blocked by the glycolytic inhibitor 2-deoxyglucose. Am J Physiol Regul Integr Comp Physiol 314, R135–R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE, 2000. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci 20, 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamounas LA, Blue ME, Siuciak JA, Altar CA, 1995. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J Neurosci 15, 7929–7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Sieck GC, 2009. Retrograde labeling of phrenic motoneurons by intrapleural injection. J Neurosci Methods 182, 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos LL, Trufelli DC, de Matos MG, da Silva Pinhal MA, 2010. Immunohistochemistry as an important tool in biomarkers detection and clinical practice. Biomark Insights 5, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB, 2001. Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol (1985) 90, 2466–2475. [DOI] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Dougherty BJ, Mitchell GS, 2017. Enhanced recovery of breathing capacity from combined adenosine 2A receptor inhibition and daily acute intermittent hypoxia after chronic cervical spinal injury. Exp Neurol 287, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Mitchell GS, 2014a. Recruitment and plasticity in diaphragm, intercostal, and abdominal muscles in unanesthetized rats. J Appl Physiol (1985) 117, 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Mitchell GS, 2014b. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol 307, R1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Vinit S, Dougherty BJ, Mitchell GS, 2015. Daily acute intermittent hypoxia elicits functional recovery of diaphragm and inspiratory intercostal muscle activity after acute cervical spinal injury. Exp Neurol 266, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NSCISC, 2020. The University of Alabama at Birmingham: National Spinal Cord Injury Statistical Center. [Google Scholar]
- Perrier JF, 2016. Modulation of motoneuron activity by serotonin. Dan Med J 63. [PubMed] [Google Scholar]
- Perrier JF, Rasmussen HB, Christensen RK, Petersen AV, 2013. Modulation of the intrinsic properties of motoneurons by serotonin. Curr Pharm Des 19, 4371–4384. [DOI] [PubMed] [Google Scholar]
- Perrin FE, Noristani HN, 2019. Serotonergic mechanisms in spinal cord injury. Exp Neurol 318, 174–191. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, de Castro D, Llewellyn-Smith I, Lipski J, Voss MD, 1990. Serotonin immunoreactive boutons make synapses with feline phrenic motoneurons. J Neurosci 10, 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankari A, Bascom A, Oomman S, Badr MS, 2014. Sleep disordered breathing in chronic spinal cord injury. J Clin Sleep Med 10, 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankari A, Vaughan S, Bascom A, Martin JL, Badr MS, 2019. Sleep-Disordered Breathing and Spinal Cord Injury: A State-of-the-Art Review. Chest 155, 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saruhashi Y, Young W, Perkins R, 1996. The recovery of 5-HT immunoreactivity in lumbosacral spinal cord and locomotor function after thoracic hemisection. Exp Neurol 139, 203–213. [DOI] [PubMed] [Google Scholar]
- Satriotomo I, Dale EA, Dahlberg JM, Mitchell GS, 2012. Repetitive acute intermittent hypoxia increases expression of proteins associated with plasticity in the phrenic motor nucleus. Exp Neurol 237, 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seven YB, Perim RR, Hobson OR, Simon AK, Tadjalli A, Mitchell GS, 2018. Phrenic motor neuron adenosine 2A receptors elicit phrenic motor facilitation. J Physiol 596, 1501–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skagerberg G, Björklund A, 1985. Topographic principles in the spinal projections of serotonergic and non-serotonergic brainstem neurons in the rat. Neuroscience 15, 445–480. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW, 1981. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 6, 557–618. [DOI] [PubMed] [Google Scholar]
- Tai Q, Palazzolo KL, Goshgarian HG, 1997. Synaptic plasticity of 5-hydroxytryptamine-immunoreactive terminals in the phrenic nucleus following spinal cord injury: a quantitative electron microscopic analysis. J Comp Neurol 386, 613–624. [PubMed] [Google Scholar]
- Törk I, 1990. Anatomy of the serotonergic system. Ann N Y Acad Sci 600, 9–34; discussion 34-35. [DOI] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J, 2003. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil 82, 803–814. [DOI] [PubMed] [Google Scholar]
- Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM, 2008. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 31, 1071–1078. [PMC free article] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG, 2000. 5-Hydroxytryptophan-induced respiratory recovery after cervical spinal cord hemisection in rats. J Appl Physiol (1985) 89, 1528–1536. [DOI] [PubMed] [Google Scholar]