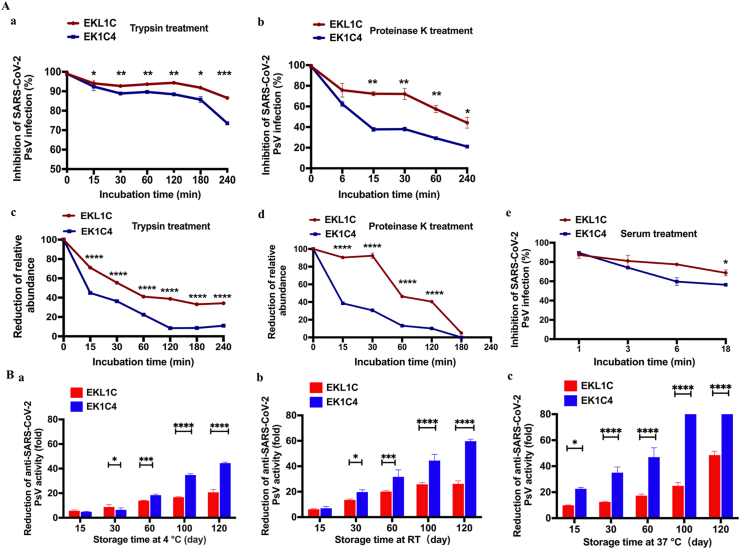
Figure 5.
Comparison of the sensitivity to proteolytic enzymes (trypsin and proteinase K), metabolic stability in mouse serum, and thermostability between EKL1C and EK1C4. (A) The sensitivity of EKL1C and EK1C4 to trypsin (a, c) and proteinase K (b, d) after the peptides were treated with trypsin or proteinase K at 37 °C for different time as determined by SARS-CoV-2 PsV inhibition assay (a, b) and LC–MS quantitative analysis (c, d), respectively. The metabolic stability of EKL1C and EK1C4 in mouse serum was assessed by SARS-CoV-2 PsV inhibition assay (e). Data are presented as mean ± SD (n = 3). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001 vs. EK1C4 groups. (B) Thermostability of EKL1C and EK1C4 stored at 4 °C for 120 days (a), RT for 120 days (b), and at 37 °C for 120 days (c). The inhibitory activity of the peptides against SARS-CoV-2 PsV infection was detected. Data are presented as mean ± SD (n = 3). ∗P < 0.05, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.