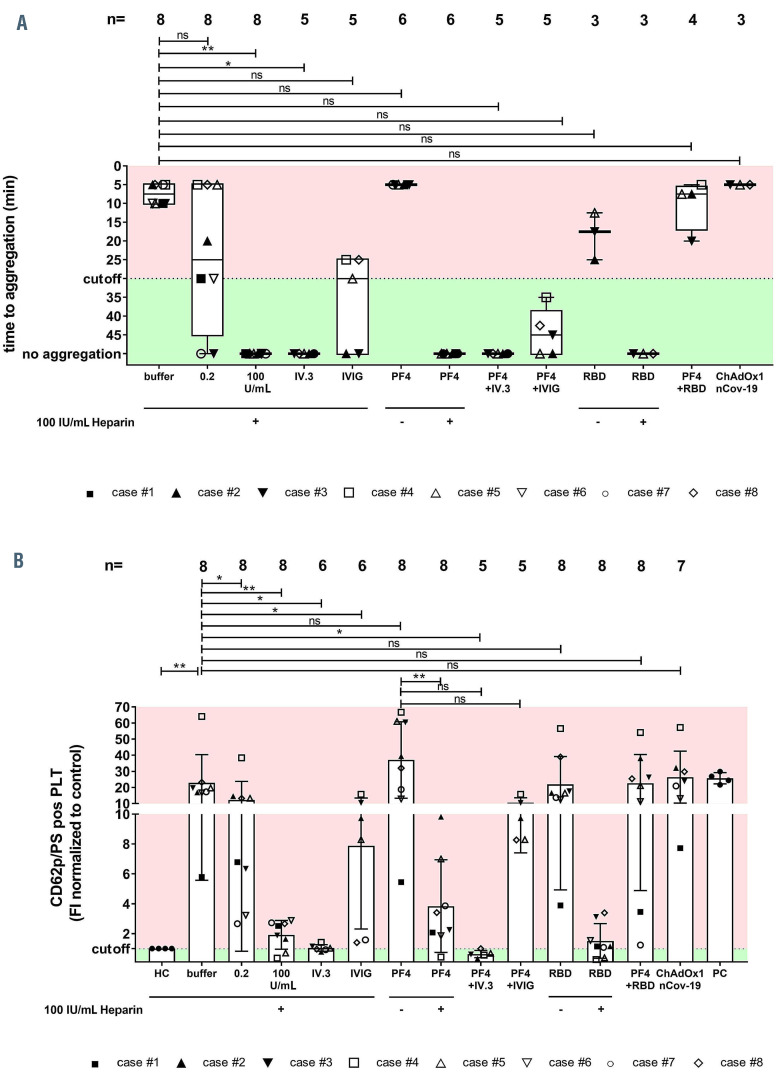
Figure 6.
Antibody-mediated platelet activation and generation of procoagulant platelets. Results of the platelet activation assay (HIPA) with modifications in the vaccine-induced immune thrombotic thrombocytopenia (VITT) patients. Each dot represents the median of four different donors. (A) All VITT patients presented platelet (PLT) activation with buffer alone, which was significantly increased by PF4 but inhibited with high dose of heparin. Procoagulant platelets (CD62P/phosphatidylserine [PS] positive) in different settings were analyzed via Annexin V-FITC and CD62p-APC antibody staining. (B) Where indicated, platelets were treated with PF4, 0.2 U/mL and 100 IU/mL heparin, RBD and ChAdOx1 nCoV-19A. Data are presented as mean ± standard deviation of the measured fold increase compared to control. ns: not significant; *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. The number of sera tested is reported in each graph. Dotted lines represent the cutoffs determined testing sera from healthy donors. FI: fold increase.