Abstract
Scrub typhus and other rickettsial infections contribute to 25 – 50% of acute undifferentiated febrile illnesses in endemic regions. Delayed recognition and therapy increase the morbidity and mortality. The constellation of fever with eschar or rash and multisystem involvement should facilitate the diagnosis and initiation of appropriate therapy. The pathological hallmark of rickettsial infections is endothelial infection and inflammation causing vasculitis. Endothelial inflammation results in microvascular dysfunction and increased vascular permeability. Immune and endothelial activation may worsen microvascular dysfunction, predisposing to multi-organ failure. Serology is the mainstay of diagnosis, although false negatives occur early in the disease. Point-of-care rapid diagnostic tests and molecular techniques, such as quantitative polymerase chain reaction (qPCR), can hasten diagnostic processes. Intravenous doxycycline with a loading dose is the most widely used antibiotic in critically ill patients, with azithromycin as a suitable alternative. Early appropriate treatment and organ support can decrease the duration of illness and be life-saving.
How to cite this article: Gunasekaran K, Bal D, Varghese GM, et al. Scrub Typhus and Other Rickettsial Infections. Indian J Crit Care Med 2021;25(Suppl 2):S138–S143.
Keywords: Rickettsial diseases, Scrub typhus, Spotted fever
Introduction
Rickettsial infections, caused by obligate intracellular bacteria within two genera, Orientia and Rickettsia of the family Rickettsiaceae, are zoonotic illnesses with similar clinical features but distinct etiologies and epidemiology. Humans acquire infections caused by these bacteria when their respective arthropod vectors feed on them. Of the rickettsial infections, scrub typhus is the commonest worldwide, causing an estimated 1,000,000 cases per year. It is also the most important clinically due to its higher case fatality rate.1
Rickettsial infections cause high morbidity and mortality, despite the widespread availability of low-cost, effective antimicrobial therapy due to delays in diagnosis and treatment. The primary reason for this is that in regions where these rickettsial infections are endemic, dengue, malaria, enteric fever, leptospirosis, and other acute undifferentiated febrile illnesses are common and present with similar clinical manifestations.2 Secondly, the lack of point-of-care diagnostic tests adds to the difficulty in reaching an early and accurate diagnosis. Thirdly, the initial symptoms of these infections seem benign and do not always prompt quick action, allowing rapid progression to multi-organ dysfunction and death.3
Organ injury in rickettsial infections results from microvascular dysfunction. This is caused by endothelial infection and inflammation, the hallmark of rickettsial infections. Endothelial inflammation leads to endothelial and platelet activation that causes platelet adhesion, microvascular thrombosis, and dysfunction, causing multi-organ failure. The common complications of scrub typhus due to endothelial inflammation include hepatitis, acute respiratory distress syndrome (ARDS), renal failure, myocarditis, septic shock, and meningoencephalitis.4 Once multi-organ involvement develops in rickettsial infections, the course of illness can be severe, life-threatening, and fulminant, particularly in scrub typhus and spotted fever group (SFG) rickettsiosis. Multiple organ dysfunction syndromes (MODS) are reported frequently (34–50%) in patients with rickettsial infections, and 18 to 30% of these patients require ventilatory support.4,5
Multiple studies have shown that delays in the recognition and administration of appropriate antibiotic therapy increase the chance of severe disease, whereas early diagnosis and appropriate management of complications improve outcomes.5–7 Hence, in order to reduce morbidity and mortality due to rickettsial infections, there needs to be a high clinical suspicion of these infections in the presence of epidemiological data and clinical manifestations, such as rash, eschar, and multi-organ involvement. Further, laboratory clues, such as elevated liver enzymes, thrombocytopenia, and normal or mildly elevated white blood counts, should prompt the diagnosis of rickettsial infections, and appropriate treatment needs to be initiated as soon as possible.
This review provides a comprehensive overview of scrub typhus and other rickettsial infections along with their clinical manifestations, as well as the relevant diagnostic tests and treatment measures currently available.
Classification of Rickettsial Infections
Rickettsial infections were originally classified clinically into the “spotted fever,” “typhus,” and “scrub typhus” groups. In 1995, 16s rRNA gene sequencing of Rickettsia tsutsugamushi showed significant differences between this microorganism and other Rickettsia, resulting in its re-assignment to the genus Orientia.8
A new genetic classification scheme, using whole-genome sequencing data, divides the Rickettsiae into four groups. These include the spotted fever group with Rickettsia conorii, Rickettsia rickettsii, and several others; the typhus group that includes Rickettsia typhi and Rickettsia prowazekii; the ancestral group with Rickettsia canadensis and Rickettsia bellii; and the transitional group that was recently created with Rickettsia australis, Rickettsia akari, and Rickettsia felis.9 In addition to these groups, several newer or re-emerging Rickettsial infections have also been described over the past few decades. These include tick-borne lymphadenopathy, Rickettsia sibirica infection causing lymphangitis-associated rickettsiosis, and Rickettsia slovaca infection causing Dermacentor-borne necrosis eschar lymphadenopathy (DEBONEL). Among the various rickettsial infections, the most common ones in India are scrub typhus and spotted fever caused by Orientia tsutsugamushi and R. conorii, respectively.
Humans contract rickettsial infections from arthropods. In nature, rickettsia are usually maintained in small mammals, such as rodents, with arthropod vectors acting as reservoirs or amplifiers. In their vector hosts, rickettsia are maintained for a long period of time, multiply efficiently, and are transmitted by transovarial and transstadial transmission. While human hosts acquire tick-borne rickettsial infections via salivary secretions from ticks, they acquire flea and louse-borne rickettsial infections when broken skin or mucosa is contaminated by infected vector feces.
The classification, vectors, and clinical manifestations of common rickettsial infections are summarized in Table 1.
Table 1.
Classification, distribution, vector, clinical features of some common rickettsial infections
| Group | Organism | Disease | Geographic distribution | Vector | Clinical features | Rash | Eschar | Case fatality rate |
|---|---|---|---|---|---|---|---|---|
| Scrub typhus group | Orientia tsutsugamushi | Scrub typhus | Asia, Northern Australia, Pacific and Indian Ocean islands | Mite | Fever, headache, myalgia, breathlessness | <50%; maculopapular rash; centrifugal | 40–90% | 5–25% |
| Rickettsia prowazekii | Epidemic typhus (louse-borne typhus) | Worldwide | Louse | Fever, headache, myalgia, rash, severe illness if untreated | 20–80%; macular; centrifugal spread | None | 6–30% | |
| Typhus group | Rickettsia typhi | Murine typhus or endemic typhus | Worldwide | Flea | Fever, headache, myalgia, rash, milder illness than epidemic typhus | 50% maculopapular rash on the trunk | None | 0–0.4% |
| Rickettsia rickettsii | Rocky Mountain spotted fever | United States, South America | Tick | Fever, headache, malaise, nausea, vomiting, abdominal pain. | >90%; macular; centripetal spread | None | 10–25% | |
| Spotted fever group | Rickettsia conorii | Indian tick typhus or Mediterranean spotted fever | Mediterranean countries; Africa; Middle East; India | Tick | Fever, headache, rash, vomiting | >90%; maculopapular rash; centripetal spread from extremities | 50% | 6–32% |
| Rickettsia sibirica | Siberian tick typhus | Northern and Central Asia | Tick | Fever, headache, rash, eschar | >90%, maculopapular, petechial or purpuric | >90% | low | |
| Rickettsia akari | Rickettsial pox | United States; Russia; Korea; South Africa | Mite | Fever, headache, vesicular rash, photophobia | 100%; papulo-vesicular | 90% | low | |
| Transitional group | Rickettsia australis | Queensland tick typhus | Queensland tick typhus | Tick | Fever, rash of trunks and limbs | Maculopapular, petechial, or vesicular | 50–66% | 3% |
Pathophysiology of Rickettsial Infections
The pathological hallmark of rickettsial infections is endothelial infection and inflammation.10,11 Direct bacterial invasion of the endothelial cells causes vasculitis and drives the pathological process. Endothelial involvement in several organs, including skin, lung, liver, kidney, heart, and brain, has been reported in human postmortem studies.12
Additionally, the immune-mediated host response also contributes to the pathology. The main target cells of rickettsiae after inoculation into the skin are the dermal dendritic cells and activated monocytes or macrophages. These cells stimulate cell signaling cascades, leading to the secretion of cytokines and chemokines. The endothelial inflammation results in microvascular dysfunction leading to increased vascular permeability, which causes acute lung injury and shock. Furthermore, the immune and endothelial activation may lead to platelet adhesion and microvascular thrombosis, worsening the microvascular dysfunction, predisposing the individual to multi-organ failure. Subsequent damage to cells and increased vascular permeability lead to edema, hypovolemia, hypotension, reduced perfusion of organs, and organ failure.
Clinical Manifestations
Rickettsial infections are acute febrile illnesses manifesting with a wide range of symptoms, including fever, headache, breathlessness, cough, nausea, vomiting, and myalgia. Skin manifestations are also often seen in rickettsial infections.
A variable proportion of patients with scrub typhus develop a characteristic eschar at the site of inoculation on the skin, which is typically seen at thin, moist skin folds, including the neck, axilla, waist, groin, and inguinal area (Fig. 1). While the symptoms take 10 – 12 days for the chigger bite to appear, the incubation period can be anywhere between 6 days and 21 days.13
Fig. 1.
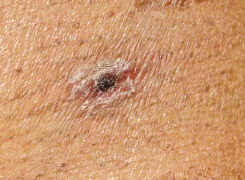
A typical eschar seen in scrub typhus patients
Among spotted fever cases, the skin rash is considered a hallmark finding, developing usually 3 – 5 days from the onset of fever, with maculopapular or occasionally vesicular manifestations, classically on the lower legs, ankle, wrists, palms, and soles, which can spread centripetally to involve the entire body.14 It is important to appreciate that all cases may not present with a rash (sometimes called “spotless spotted fever”). Typically, the rash, which is erythematous and blanching initially, progresses into discrete macular or maculopapular rashes. It may also progress into petechial rashes or more severe hemorrhagic lesions, with ecchymosis and gangrenous patches, due to the vasculitis and thrombosis (Fig. 2).
Fig. 2.
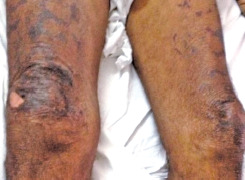
Spotted fever with purpura fulminans rash
In murine typhus, the rash is characteristically more diffuse, macular, and/or maculopapular appearing within 3 – 10 days of the onset of fever. It appears on the trunk, spreads centrifugally, sometimes including the palms and soles (a pathognomonic finding for spotted fever).
Although self-limiting cases are reported, severe disease with multiple organ involvement is reported in a large proportion of rickettsial infections frequently due to delays in the diagnosis and administration of appropriate antibiotic therapy.
In scrub typhus, complications usually develop after a week of illness. Hepatitis, pneumonitis, ARDS, myocarditis, meningoencephalitis, and acute renal failure are common. More than a third of hospitalized patients develop ARDS; and the majority of them need ventilator support.4,15 The common radiological findings include focal infiltrates, thickening around the bronchi, pulmonary edema, reticulonodular opacities, and findings suggestive of ARDS.16 Although pulmonary involvement indicates a more severe disease, dramatic recovery and rapid weaning of patients on the ventilator with ARDS after initiation of appropriate antibiotics have been reported.13
Severe spotted fever can also manifest with MODS, bronchopneumonia, ARDS, myocarditis, and heart failure. Notably, an overt vasculitis that results in gangrene of toes and fingers requiring amputation is described in spotted fever when the diagnosis is delayed.17 Louse-borne epidemic typhus can also cause complications, such as gangrene, parotitis, otitis, and myopericarditis.18
In scrub typhus, cardiac involvement with myocarditis and heart failure is common.19 Electrocardiogram commonly shows sinus tachycardia. Other abnormalities, such as ST–T changes, PR-interval prolongation, Q–T prolongation, relative bradycardia, atrial flutter or fibrillation, ventricular premature beats, and supraventricular tachycardia, are also described. Pericarditis and myocardial infarction have also been reported.13
Hepatic dysfunction is well-documented in scrub typhus. Modest elevation of transaminases is very common in more than 80% of cases, but bilirubin elevation is seen in only 16 – 38% of cases.4,15 However, liver failure is very rare.
Acute kidney injury occurs in 12 – 35% of patients with scrub typhus and is usually mild and without oliguria. About 5 to 10% require hemodialysis;20,21 however, in most patients, there is complete renal function recovery. Acute renal failure may develop due to vasculitis, disseminated intravascular coagulation (DIC), or prerenal azotemia. Renal tubular damage from direct invasion has also been demonstrated in autopsy specimens.
The commonest neurological manifestation in scrub typhus is meningitis or meningoencephalitis occurring in about 20% of cases.4,15 Focal neurological deficits are rare; however, palsies of the second, third, sixth, seventh, and eighth cranial nerves occasionally occur. Rarer manifestations include cerebellitis and acute disseminated encephalomyelitis. Parkinsonism, neuropsychiatric abnormality, cerebral vein thrombosis, cerebrovascular events, opsoclonus and myoclonus, Guillain–Barre syndrome, transverse myelitis, plexopathy, and peripheral neuropathy are also seen. Most patients with neurological involvement have increased protein and lymphocyte-predominant pleocytosis on cerebrospinal fluid analysis.13
MODS is seen in about a third of patients with scrub typhus. Griffith et al. in their study of patients with severe scrub typhus requiring intensive care unit (ICU) care report that nearly 80% of patients had dysfunctions of more than three organ systems.7 They found that the admission APACHE score and duration of illness were independent predictors of mortality. However, the mortality in this cohort of severe scrub typhus patients was 24%, which was lower than the predicted mortality based on the organ dysfunction and high admission APACHE scores in the study.
Diagnosis
The challenges in diagnosing rickettsial infections include the nonspecific clinical features, paucity of good point-of-care tests, and false negativity of serological tests in the first week of illness.
A few laboratory parameters set scrub typhus apart from other tropical infections, including leukocytosis (seen in about 50% of patients) and thrombocytopenia (80%). However, initially, the total leukocyte count may be normal or even be low. Transaminases are often mildly elevated (80%) and about double or triple the value of the normal upper limit. Chest X-ray abnormality with multisystem involvement could be a clue to scrub typhus and other rickettsial infections.
In clinical practice, serological tests are most useful for diagnostic confirmation. Enzyme-linked immunosorbent assay (ELISA), immunochromatographic rapid diagnostic tests (RDT), and indirect immunofluorescent assay (IFA) are central to the diagnosis of scrub typhus. Detection of IgM by these tests is found to have good sensitivity and specificity. In a recent study evaluating various tests for the diagnosis of scrub typhus, ELISA was found to have a sensitivity of 94.2% and a specificity of 93.6%, making it very reliable.22 The accuracy of IFA, however, was found to be suboptimal. Although considered the standard reference serological test in the past, it requires expensive equipment to do, is tedious, and has interobserver variations. As the scrub typhus ELISA is accurate and has become commonly available, more feasible, and cheaper, it is now the most widely used confirmatory test. In primary and secondary health-care settings, new RDT, such as the ImmuneMed scrub typhus rapid, with good sensitivity and specificity, may prove useful in diagnosing scrub typhus. The Weil-Felix test, which has very low sensitivity, has become obsolete.
The quantitative polymerase chain reaction (qPCR) on blood buffy coat targeting the 47-kDa gene has excellent sensitivity (97%) and perfect specificity22, whereas loop-mediated isothermal amplification assay and conventional PCR perform suboptimally. Although qPCR is expensive and done only in well-equipped laboratories, it can be valuable in the first week of disease when serological tests are still negative.
Spotted fever rickettsiosis is confirmed by PCR, IgM or IgG serology, antigen on immunohistochemistry, or organism in cell culture. Since the culture of the organism is cumbersome, a molecular test like PCR is considered an effective confirmatory test. The use of ompA qPCR, as molecular amplification of ompA gene, is confirmatory in spotted fever rickettsiosis.23
Treatment
Scrub typhus and other rickettsial infections respond promptly to treatment with appropriate antibiotics, such as doxycycline, azithromycin, and chloramphenicol. Doxycylcine and azithromycin can be used to treat mild scrub typhus. However, there is no clarity on the optimal drug treatment in severe disease.24,25 Currently, a large randomized control trial is being conducted to assess how efficacious different antibiotics are in treating severe disease.26
The most commonly used antibiotic for mild rickettsial infections is twice-daily dosing of 100 mg of doxycycline. Intravenous doxycycline has not been freely available in many endemic countries, and there has been a significant concern of bioavailability of oral preparations for treating severe life-threatening scrub typhus. Further, gastrointestinal absorption of the oral preparation in patients with MODS is unpredictable, especially in patients with hypoalbuminemia and diminished gastrointestinal perfusion due to shock or the use of vasopressors. The recommended dosage of intravenous doxycycline (100 mg twice daily) may result in lower exposure of the drug in patients with shock in whom the absorption and volume of distribution of the drug may be altered. Hence, a loading dose of intravenous doxycycline is commonly used. This has not yet been proven to be effective and needs exploration through prospective studies.
Azithromycin is considered a safe alternative to doxycycline and is the preferred treatment in pregnant women with scrub typhus.27 Azithromycin can be given both orally and intravenously (500 mg once daily for 5–7 days) in mild scrub typhus. The role of azithromycin in treating severe scrub typhus is being evaluated currently.
Chloramphenicol (250–500 mg intravenously or orally every 6 hours) has long been considered the main alternative agent for treating rickettsial infections. However, its use is limited because of its potential to cause bone marrow toxicities, such as aplastic anemia and acute hemolytic anemia in patients with G6PD deficiency, even though these are rare complications and regular complete blood count examinations could help us identify these early. Chloramphenicol is also contraindicated in the third trimester of pregnancy due to the possibility of “grey baby” syndrome. In vitro and in vivo data indicate that chloramphenicol is less effective than tetracycline.28,29 Further, in children with Rocky Mountain spotted fever, the prognosis is significantly worse with chloramphenicol.30
Rifampin has been studied in SFG and scrub typhus. A randomized trial from Thailand shows that treatment with rifampicin (300–450 mg twice daily for 1 week) is effective as treatment.31 However, since tuberculosis is prevalent in many areas where scrub typhus is endemic and requires prolonged treatment, it may be better to avoid using rifampicin to treat scrub typhus in order to prevent inducing resistant tuberculosis.
Fluoroquinolones with anti-rickettsial activity have been studied in Mediterranean spotted fever and demonstrate resolution of symptoms.32,33 However, their use is limited since they are not very effective in scrub typhus and are contraindicated in pregnancy.
Apart from antibiotic therapy, patients with severe disease and various organ involvement carry risks of higher mortality and will require intensive organ support based on the extent of organ dysfunction. Management of respiratory failure and ARDS is key in the treatment of seriously ill patients to improve their outcomes. Patients with respiratory failure require invasive or noninvasive ventilation as per standard treatment protocol in the ICU. Those with shock are managed with fluid resuscitation and vasopressor support. Acute renal failure may require renal replacement therapy. Those with DIC and DIC-like syndromes with other coagulopathies will require support with blood products in case of clinical bleeding.
Conclusion
Scrub typhus and other rickettsial infections are an important group of under-diagnosed and under-recognized re-emerging vector-borne diseases worldwide. The constellation of symptoms consisting of fever with eschar or rash and multisystem involvement should facilitate clinical diagnosis and guide the early initiation of appropriate therapy. Serological testing is the mainstay of diagnosis for rickettsial diseases. RDT and molecular techniques, such as PCR, have hastened the diagnostic process in appropriate settings. Intravenous doxycycline with a loading dose is the most widely used antibiotic in critically ill patients, with azithromycin as a suitable alternative. Early and appropriate treatment can decrease the duration of illness and can be life-saving.
Acknowledgments
We gratefully acknowledge Dr. Divya Dayanand and Dr. Yvonne Schmiedel for reviewing the manuscript.
Orcid
Karthik Gunasekaran https://orcid.org/0000-0002-8615-5753
Deepti Bal https://orcid.org/0000-0001-6184-7422
George M Varghese https://orcid.org/0000-0002-4040-5649
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.John R, Varghese GM. Scrub typhus: a reemerging infection. Curr Opin Infect Dis. 2020;33(5):365–371. doi: 10.1097/QCO.0000000000000664. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 2.The Indian Society of Critical Care Medicine Tropical fever Group. Singhi S, Chaudhary D, Varghese GM, Bhalla A, Karthi N, et al. Tropical fevers: management guidelines. Indian J Crit Care Med. 2014;18(2):62–69. doi: 10.4103/0972-5229.126074. DOI: PMID: PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9(8):e0003971. doi: 10.1371/journal.pntd.0003971. DOI: PMID: PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varghese GM, Trowbridge P, Janardhanan J, Thomas K, Peter JV, Mathews P, et al. Clinical profile and improving mortality trend of scrub typhus in South India. Int J Infect Dis. 2014;23:39–43. doi: 10.1016/j.ijid.2014.02.009. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 5.Bagshaw RJ, Stewart AGA, Smith S, Carter AW, Hanson J. The characteristics and clinical course of patients with scrub typhus and queensland tick typhus infection requiring intensive care unit admission: a 23-year case series from Queensland, Tropical Australia. Am J Trop Med Hyg. 2020;103(6):2472–2477. doi: 10.4269/ajtmh.20-0780. DOI: PMID: PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkland KB, Wilkinson WE, Sexton DJ. Therapeutic delay and mortality in cases of Rocky Mountain spotted fever. Clin Infect Dis. 1995;20(5):1118–1121. doi: 10.1093/clinids/20.5.1118. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 7.Griffith M, Peter JV, Karthik G, Ramakrishna K, Prakash JA, Kalki RC, et al. Profile of organ dysfunction and predictors of mortality in severe scrub typhus infection requiring intensive care admission. Indian J Crit Care Med. 2014;18(8):497–502. doi: 10.4103/0972-5229.138145. DOI: PMID: PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamura A, Ohashi N, Urakami H, Miyamura S. Classification of Rickettsia tsutsugamushi in a new genus, Orientia gen. nov., as Orientia tsutsugamushi comb. nov. Int J Syst Bacteriol. 1995;45(3):589–591. doi: 10.1099/00207713-45-3-589. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 9.Kocher C, Morrison AC, Leguia M, Loyola S, Castillo RM, Galvez HA, et al. Rickettsial disease in the Peruvian Amazon Basin. PLoS Negl Trop Dis. 2016;10(7):e0004843. doi: 10.1371/journal.pntd.0004843. DOI: PMID: PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Díaz FE, Abarca K, Kalergis AM. An update on host-pathogen interplay and modulation of immune responses during Orientia tsutsugamushi infection. Clin Microbiol Rev. 2018;31(2):e00076–17. doi: 10.1128/CMR.00076-17. DOI: PMID: PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salje J. Cells within cells: Rickettsiales and the obligate intracellular bacterial lifestyle. Nat Rev Microbiol. 2021 doi: 10.1038/s41579-020-00507-2. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 12.Moron CG, Popov VL, Feng HM, Wear D, Walker DH. Identification of the target cells of Orientia tsutsugamushi in human cases of scrub typhus. Mod Pathol. 2001;14(8):752–759. doi: 10.1038/modpathol.3880385. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 13.Rajapakse S, Weeratunga P, Sivayoganathan S, Fernando SD. Clinical manifestations of scrub typhus. Trans R Soc Trop Med Hyg. 2017;111(2):43–54. doi: 10.1093/trstmh/trx017. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 14.Faccini-Martínez ÁA, García-Álvarez L, Hidalgo M, Oteo JA. Syndromic classification of rickettsioses: an approach for clinical practice. Int J Infect Dis. 2014;28:126–139. doi: 10.1016/j.ijid.2014.05.025. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 15.Varghese GM, Janardhanan J, Trowbridge P, Peter JV, Prakash JA, Sathyendra S, et al. Scrub typhus in South India: clinical and laboratory manifestations, genetic variability, and outcome. Int J Infect Dis. 2013;17(11):e981–e987. doi: 10.1016/j.ijid.2013.05.017. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 16.Abhilash K, Mannam PR, Rajendran K, John RA, Ramasami P. Chest radiographic manifestations of scrub typhus. J Postgrad Med. 2016;62(4):235–238. doi: 10.4103/0022-3859.184662. DOI: PMID: PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkland KB, Marcom PK, Sexton DJ, Dumler JS, Walker DH. Rocky Mountain spotted fever complicated by gangrene: report of six cases and review. Clin Infect Dis. 1993 May;16(5):629–34. doi: 10.1093/clind/16.5.629. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 18.Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16(5):429–436. doi: 10.1097/00001432-200310000-00009. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 19.Karthik G, Sudarsan TI, Peter JV, Sudarsanam T, Varghese GM, Kundavaram P, et al. Spectrum of cardiac manifestations and its relationship to outcomes in patients admitted with scrub typhus infection. World J Crit Care Med. 2018;7(1):16–23. doi: 10.5492/wjccm.v7.i1.16. DOI: PMID: PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sedhain A, Bhattarai GR. Renal manifestation in scrub typhus during a major outbreak in Central Nepal. Indian J Nephrol. 2017;27(6):440–445. doi: 10.4103/ijn.IJN_133_17. DOI: PMID: PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attur RP, Kuppasamy S, Bairy M, Nagaraju SP, Pammidi NR, Kamath V, et al. Acute kidney injury in scrub typhus. Clin Exp Nephrol. 2013;17(5):725–729. doi: 10.1007/s10157-012-0753-9. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 22.Kannan K, John R, Kundu D, Dayanand D, Abhilash KPP, Mathuram AJ, et al. Performance of molecular and serologic tests for the diagnosis of scrub typhus. PLoS Negl Trop Dis. 2020;14(11):e0008747. doi: 10.1371/journal.pntd.0008747. DOI: aPMID: PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aung AK, Spelman DW, Murray RJ, Graves S. Rickettsial infections in Southeast Asia: implications for local populace and febrile returned travelers. Am J Trop Med Hyg. 2014;91(3):451–460. doi: 10.4269/ajtmh.14-0191. DOI: PMID: PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SC, Cheng YJ, Lin CH, Lei WT, Chang HY, Lee MD, et al. Comparative effectiveness of azithromycin for treating scrub typhus: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2017;96(36):e7992. doi: 10.1097/MD.0000000000007992. DOI: PMID: PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajapakse S, Rodrigo C, Fernando SD. Drug treatment of scrub typhus. Trop Doct. 2011;41(1):1–4. doi: 10.1258/td.2010.100311. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 26.http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=27483&EncHid=&userName=scrub%20typhus
- 27.Fang Y, Huang Z, Tu C, Zhang L, Ye D, Zhu BP. Meta-analysis of drug treatment for scrub typhus in Asia. Intern Med. 2012;51(17):2313–2320. doi: 10.2169/internalmedicine.51.7816. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 28.Ormsbee RA, Parker H, Pickens EG. The comparative effectiveness of aureomycin, terramycin, chloramphenicol erythromycin, and thiocymetin in suppressing experimental rickettsial infections in chick embryos. J Infect Dis. 1955;96(2):162–167. doi: 10.1093/infdis/96.2.162. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 29.Raoult D, Roussellier P, Vestris G, Tamalet J. In vitro antibiotic susceptibility of Rickettsia rickettsii and Rickettsia conorii: plaque assay and microplaque colorimetric assay. J Infect Dis. 1987;155(5):1059–1062. doi: 10.1093/infdis/155.5.1059. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 30.Holman RC, Paddock CD, Curns AT, Krebs JW, McQuiston JH, Childs JE. Analysis of risk factors for fatal Rocky Mountain spotted fever: evidence for superiority of tetracyclines for therapy. J Infect Dis. 2001;184(11):1437–1444. doi: 10.1086/324372. DOI: PMID: [DOI] [PubMed] [Google Scholar]
- 31.Phimda K, Hoontrakul S, Suttinont C, Chareonwat S, Losuwanaluk K, Chueasuwanchai S, et al. Doxycycline versus azithromycin for treatment of leptospirosis and scrub typhus. Antimicrob Agents Chemother. 2007;51(9):3259–3263. doi: 10.1128/AAC.00508-07. DOI: PMID: PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raoult D, Gallais H, De Micco P, Casanova P. Ciprofloxacin therapy for Mediterranean spotted fever. Antimicrob Agents Chemother. 1986;30(4):606–607. doi: 10.1128/aac.30.4.606. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raoult D, Zuchelli P, Weiller PJ, Charrel C, San Marco JL, Gallais H, et al. Incidence, clinical observations and risk factors in the severe form of Mediterranean spotted fever among patients admitted to hospital in Marseilles 1983–1984. J Infect. 1986;12(2):111–116. doi: 10.1016/s0163-4453(86)93508-5. DOI: [DOI] [PubMed] [Google Scholar]


