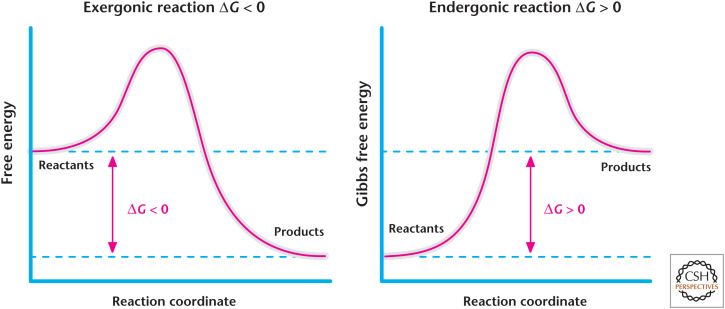
Figure 2.
Exergonic and endergonic reactions. The change in Gibbs free energy of a reaction is the energy difference between products and reactants. Exergonic reactions have a negative change in the Gibbs free energy and, thus, release energy. In contrast, endergonic reactions have a positive change in the Gibbs free energy and, thus, require energy.