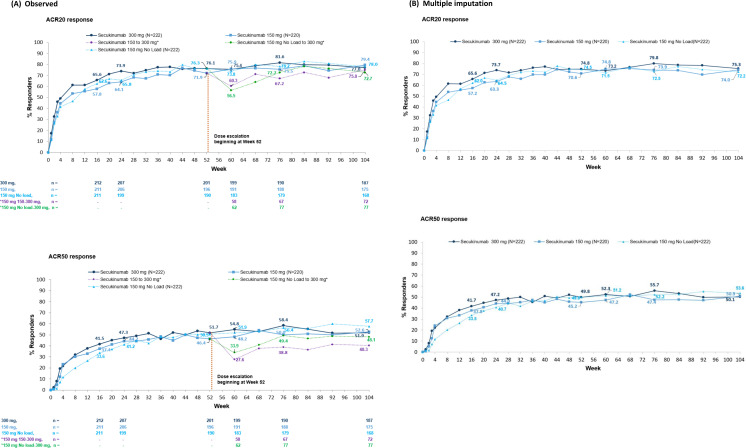
Figure 2.
ACR20 and ACR50 responses through 2 years. All data through week 104 calculated using (A) observed and (B) multiple imputation for patients originally randomised to secukinumab 300 mg, 150 mg and 150 mg no loading dose. *In observed data (A), ‘150 mg group’ included patients who were originally randomised and those who had dose escalation and ‘150 mg No load’ group included patients who were originally randomised and those who had dose escalation from week 60 to week 104, where available data after dose escalation were used (not censored). ACR, American College of Rheumatology. n, number of patients in the treatment group with ACR evaluation.