Figure 4. Transplantation of gene-corrected FP4 iPS cell-derived myogenic progenitors into FKRP mutant mice rescues functional glycosylation of α-DG.
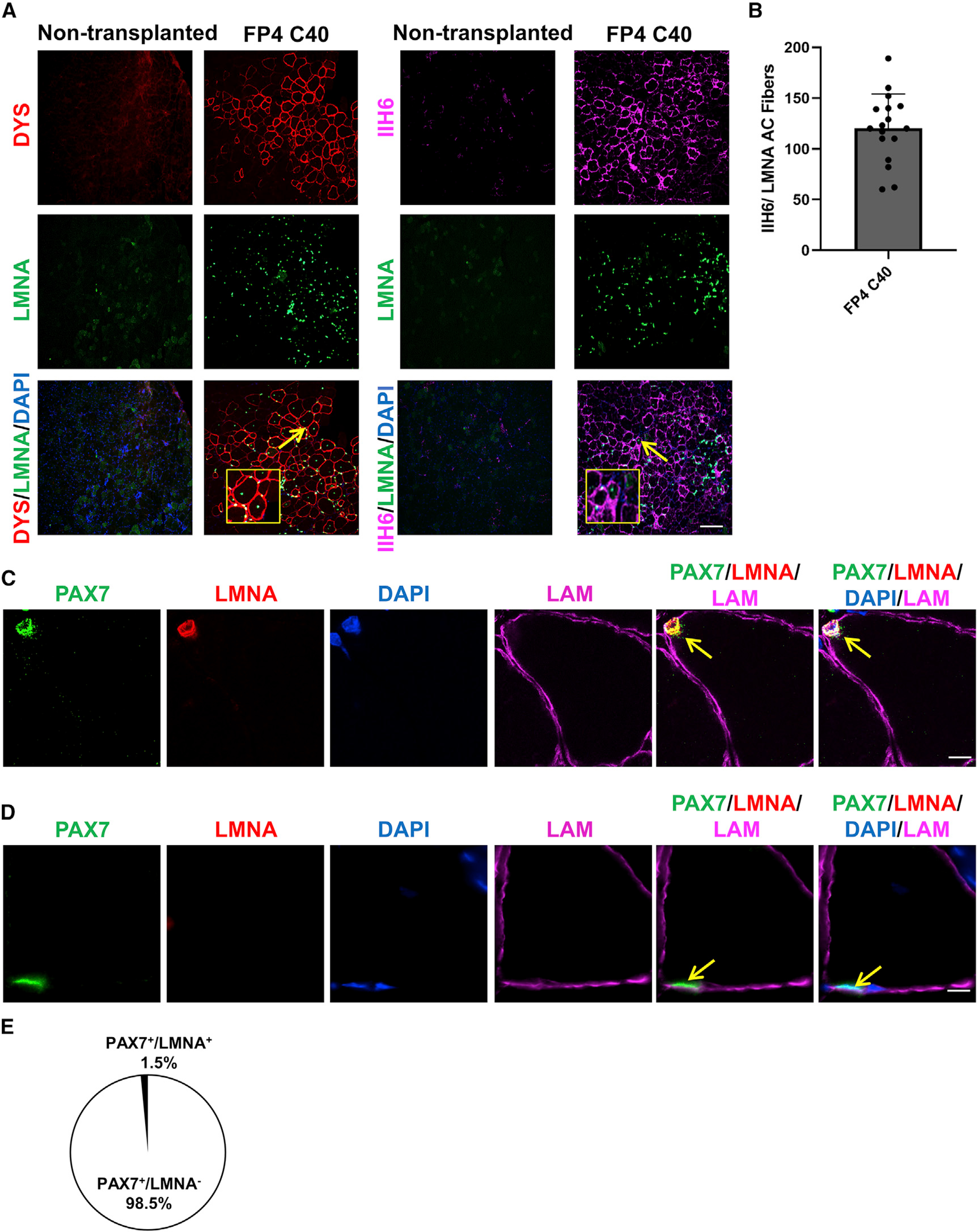
(A) Representative images show the engraftment of gene-corrected FP4 (C40) iPS cell-derived myogenic progenitors following their transplantation into TA muscles of FKRPP448L-NSG mice. The left panel shows immunostaining for human DYS (in red) and human LMNA (in green), whereas the right panel shows immunostaining for IIH6 (in purple) in combination with LMNA (in green). PBS and non-transplanted muscle served as negative controls. DAPI stains nuclei (in blue). Scale bar, 200 μm.
(B) Graph shows the total number of donor-derived myofibers, IIH6+/LMNA+, in TA muscles that had been transplanted with myogenic progenitors differentiated from gene-corrected FP4 (C40) iPS cells. Data are shown as the mean of 3 independent transplantation experiments ± SEM (n = 18 mice).
(C and D) Immunofluorescence staining for satellite cells.
(C) Representative images show donor-derived satellite cell engraftment in TA muscles transplanted with gene-edited FP4 (C40) iPS cell-derived myogenic progenitors, as shown by the presence of human LMNA+ (in red)/PAX7+ (in green) cells localized beneath the basal lamina (upper panel).
(D) Representative images indicate a recipient PAX7+/LMNA− satellite cell. Scale bar, 10 μm.
(E) Percentage of PAX7+/LMNA+ (donor-derived) and PAX7+/LMNA− (recipient-derived) cells per muscle section in mice that had been transplanted with gene-edited FP4 (C40) iPS cell-derived myogenic progenitors. Data are shown as means ± SEMs (n = 8 mice).
